
| ʹ�õ�NaOH��� | ʹ�õ�H2SO 4��� | |
| �� | NaOH���� | ϡH2SO4 |
| �� | NaOHϡ��Һ | ŨH2SO4 |
| �� | NaOHϡ��Һ | ϡH2SO4 |


 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
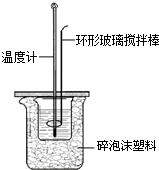
 26��ijѧϰС�����λͬѧΪ�˲ⶨH2S04��NaOH�����кͷ�Ӧʱ�ų���������������ͼ��ʾ��С�ձ���װ��һ������NaOH��Һ�������¶ȣ���ȡһ������������Һ�������¶Ⱥ��ٻ���������С�ձ��У����ӱ��û��β����������ƶ����裬��¼��Һ�¶ȵı仯���ס��ҡ�����λͬѧʵ��ʱѡ����Լ����������
26��ijѧϰС�����λͬѧΪ�˲ⶨH2S04��NaOH�����кͷ�Ӧʱ�ų���������������ͼ��ʾ��С�ձ���װ��һ������NaOH��Һ�������¶ȣ���ȡһ������������Һ�������¶Ⱥ��ٻ���������С�ձ��У����ӱ��û��β����������ƶ����裬��¼��Һ�¶ȵı仯���ס��ҡ�����λͬѧʵ��ʱѡ����Լ�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 34����ϰʱ����������ʦ�������ǻع��˶���̽��ʵ�飮
34����ϰʱ����������ʦ�������ǻع��˶���̽��ʵ�飮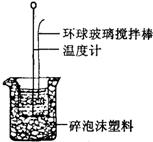 ͬѧʵ��ʱѡ����Լ����������
ͬѧʵ��ʱѡ����Լ����������| ʹ�õ�NaOH��� | ʹ�õ�H2SO 4��� | |
| �� | NaOH���� | ϡH 2SO 4 |
| �� | NaOHϡ��Һ | ŨH2SO4 |
| �� | NaOHϡ��Һ | ϡH2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ijѧϰС�����λͬѧΪ�ⶨ��п��Ƥ��п�Ʋ�ĺ�ȣ�����˸��Ե���Ʒ�����������п�Ʋ��������
ijѧϰС�����λͬѧΪ�ⶨ��п��Ƥ��п�Ʋ�ĺ�ȣ�����˸��Ե���Ʒ�����������п�Ʋ���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ijѧϰС�����λͬѧΪ�˲ⶨH2S04��NaOH�����кͷ�Ӧʱ�ų���������������ͼ��ʾ��С�ձ���װ��һ������NaOH��Һ�������¶ȣ���ȡһ������������Һ�������¶Ⱥ��ٻ���������С�ձ��У����ӱ��û��β����������ƶ����裬��¼��Һ�¶ȵı仯���ס��ҡ�����λͬѧʵ��ʱѡ����Լ����������
ijѧϰС�����λͬѧΪ�˲ⶨH2S04��NaOH�����кͷ�Ӧʱ�ų���������������ͼ��ʾ��С�ձ���װ��һ������NaOH��Һ�������¶ȣ���ȡһ������������Һ�������¶Ⱥ��ٻ���������С�ձ��У����ӱ��û��β����������ƶ����裬��¼��Һ�¶ȵı仯���ס��ҡ�����λͬѧʵ��ʱѡ����Լ����������| ʹ�õ�NaOH��� | ʹ�õ�H2SO 4��� | |
| �� | NaOH���� | ϡH2SO4 |
| �� | NaOHϡ��Һ | ŨH2SO4 |
| �� | NaOHϡ��Һ | ϡH2SO4 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com