
�����д����л�ѧ��
��1���±�ΪijƷ������Ƭ����Ҫ�ɷ֡�
| ÿ100g���е�Ӫ���ɷ� | ���� | ��֬ | ������ | ά����C | �� | �� | п |
| 7.6g | 7.8g | 7.4g | 18mg | 201mg | 30.8mg | 8.1mg |
��1��ˮ(H20) Ca ��2���黯 ��3��ѹǿ
��4�� ������̼������= 200g+20g-217.8g = 2.2g������״���CH3COOH����������Ϊ3�� ��
���������������1�����������Ӫ������Ϊˮ�������ʡ�֬����ά���ء����ࡢ�������ӣ�����ȱ��Ԫ�ػ���ɹ������ɣ���2��ϴ�Ӽ���ϴ�;��ϵ����������������黯���ã���3����������ܽ����ѹǿ�йأ���4��������̼������= 200g+20g-217.8g = 2.2g
�⣺��CH3COOH������Ϊx��
2CH3COOH + CaCO3 = (CH3COO)2Ca + CO2��+H2O
120 44
x 2.2g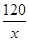 ��
�� 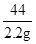 �� x��6.0 g
�� x��6.0 g 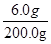 ��100% = 3��
��100% = 3��
������״���CH3COOH����������Ϊ3�� ��
���㣺��ѧ����ʽ�ļ���
���������ǻ�ѧ��Ӧ��ϵ����ʽ�ļ����⣬������Ŀ��ÿ���п���ѹ���⣬�ؿ��⣬������Ŀ���ѵ����ڰ��������߱�ʾ������������ϵ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com