ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ��װ�ú�ϰ���г���Խ��Խ�࣬����ijЩʵ������Ĺ۲��ʵ����̵ĸĽ��������벻����Ч����
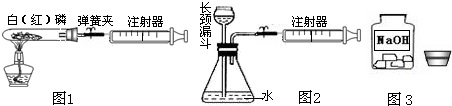
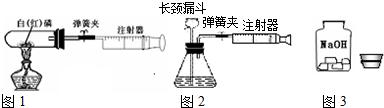
��1��ͼ1��50mL�Թ�����Ӧ��������ȼ�վ����ܱ���������У��ɷ�ֹ������Ⱦ������
ʵ��Ŀ�ģ���50mLע�������������ȴ���20mL�̶ȴ���������ȼ�����������������
�������ټ��װ�õ������ԣ���װҩƷ�������������ۼн����ɼУ����Ȱ��ף��۲��Թ�������������Ϊ
����ȼ�ս������Թ���ȴ����ɼУ����Կ��������������Ƶ�Լ
mL�̶ȴ���ȡ����ֵ����˵���������������������ԼΪ
��
��2��ͼ2������ע���������ķ������Լ��װ�õ������ԣ�������������������ʱ�����װ�����������ã����ܹ۲쵽��
��
A��ע��������Һ�壻B��ƿ��Һ��������C������©����Һ��������D������©���¶˹ܿڲ������ݣ�
��3����ȤС���߽�ʵ���ҿ�����һ������г�ġ�����������ͼ3����
�� I������˾��������뵽��ȡ�ø�ҩƷʱ�Լ�ƿƿ��Ӧ
�������ϣ�ȡ�ú�Ӧ��������ƿ���ܷⱣ�棬������Ϊ
��
��
��II������ȤС��ͬѧΪ�˲ⶨij���ʵ��ռ���Ʒ��Na
2CO
3���������������������ͼ4��ʾ��װ�ã�ͨ����Ӧ���Ҳ�ע�������ռ���������������Na
2CO
3��������������֪��״̬��CO
2���ܶȣ���ʹ��ע�����������Ϊ20mL����д����ƿ���������仯�Ļ�ѧ����ʽ��
��
��
С����Ϊ�÷�������ȷ���Na
2CO
3������������������Ϊ
����С����Ϊ�÷�������Ʒ��ȡ�õ���Ҳ��Ҫһ���Ŀ��ƣ�������Ϊ
��
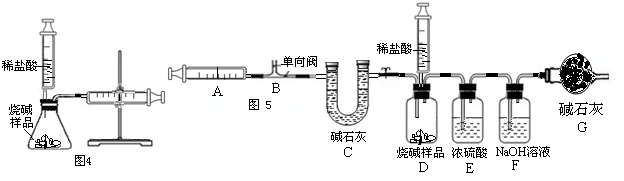
��III������ȤС��ͬѧ�ڷ�˼����ʵ����������ͼ5��װ�òⶨ���ʵ��ռ���Ʒ��Na
2CO
3��������������Ӧ�Ŀ�ʼ��������Ҫ��ע�����������������壮ʵ�鲽��Ϊ��
�ټ��װ�õ������ԣ� �ڳ�ȡ10�˸�����ռ���Ʒ������ƿ�У�D��ע�����ڼ���������ϡ���ᣬȷ����װ��F������320�ˣ�����װ�ã� �۴��ɼ�1����������ע����A 10�Σ�ȷ����װ��F������320.5�ˣ��ܹرյ��ɼ�1��Ȼ���ƶ���������ϡ������ε�����Ʒ�У�ֱ�����ٲ�������Ϊֹ�� �ݴ��ɼ�1����������ע����A 10�Σ� ����ȷ��װ��F����Ϊ321.6�ˣ�
����̽����
��i��Eװ�õ�������
��Gװ�õ�����
��
��ii�����������ע�����������ʹ��죬��ⶨ��Na
2CO
3������������
���ƫ����ƫС���������䡱����
��iii���Ը���ʵ���������ݼ�������ռ���Ʒ��Na
2CO
3����������
����Ҫ��Щ������̣���





 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д�



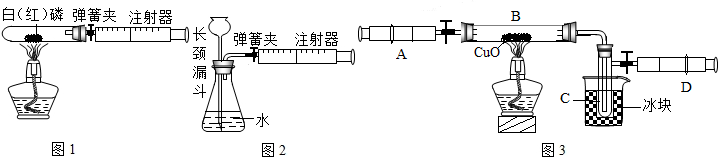
 ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����
ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����