
����ص��ܽ������ͼ��ʾ���й�������ȷ���ǣ�������
�¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 |
�ܽ�ȣ�g/100gˮ�� | 31.6 | 45.8 | 63.9 | 85.5 | 110 |
A. 20��ʱ�������50g35%������ر�����Һ
B. 30���50��ʱ��������������ر�����Һ������ˮ�������ǰ�ߴ��ں���
C. 40��ʱ��50g����ر�����Һ������20�棬�������������Ϊ32.3g
D. 60��ʱ��50g����ر�����Һ�У��������ܼ���������Ϊ11��21
 ������������ϵ�д�
������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�챱����ͨ�������꼶��һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ�������
����ʵ�鷽����Ʋ���������
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | ����NaCl��Һ��ϡ���� | �μ���ɫ��̪��Һ |
B | ����һƿ�����Ƿ�Ϊ���� | �������ǵ�ľ������ƿ�� |
C | ��ȥCO�л��е�CO2 | ���������ͨ������������Һ |
D | ��ȥ̼��Ʒ�ĩ�е�̼���ƹ��� | ������ˮ�ܽ����ˣ����º�� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ����о��꼶3��У��������ѧ�Ծ��������棩 ���ͣ������
��C��H��O��Fe����Ԫ����ɵij��г������ʼ�������ͼ��ʾ�Ĺ�ϵ������A��B��C�ǵ��ʣ��ס����ǻ����ͼ�С�������ʾ��������������֮����Է�����Ӧ����������ʾ��ijһ���ʿ��Ƶ���һ���ʡ����ַ�Ӧ������P��Ӧ������ȥ����ش��������⣺

��д���������ʵĻ�ѧʽ��A______��C______����______��
��д��A��B��Ӧ�Ļ�ѧ����ʽ_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡ��ҵ��ѧҵ����ģ��һ��ѧ�Ծ��������棩 ���ͣ������
��ͼΪij��Ƭ����Ʒ��ǩ������ݱ�ǩ���й���Ϣ������и��⣺

��1����Ҫ�ɷ�̼��ƣ�CaCO3����_______��Ԫ����ɣ�
��2��̼��Ƶ���Է���������Ϊ_______��
��3��̼����и�Ԫ�ص���������_________��
��4��̼����иơ�̼����Ԫ�ص�������Ϊ_____________��
��5��ij��ͯ����ǩ������ҩ��ÿ�첹���Ԫ��������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡ��ҵ��ѧҵ����ģ��һ��ѧ�Ծ��������棩 ���ͣ���Ϣ������
ʵ���г���Ҫ�õ�����������Һ������������Һ��¶�п������ױ��ʣ����ʺ����ҺΪ__________��Һ������ʺ����Һ�м����Ȼ�����Һ�ܹ��۲쵽��������____________���йصķ���ʽΪ:_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡ��ҵ��ѧҵ����ģ��һ��ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ��������˵��װ�����������õ��ǣ�������
A�� B��
B��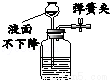
C��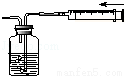 D��
D��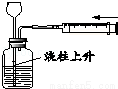
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������о��꼶�п�ģ�⻯ѧ�Ծ��������棩 ���ͣ������
��CaCl2��NaCl��������14 g�����ձ��У�����96 g̼������Һǡ����ȫ��Ӧ������Ӧ����ˣ������ձ�����Һ��������Ϊ100 g������㣺
(1)���ɳ�����������_____��
(2)�������Ȼ��Ƶ������Ƕ��٣�
(3)������Һ�����ʵ����������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������о��꼶�п�ģ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ�������
�������ʾ����ڿ����У����������仯���������ٵ��ǣ� ��
A. Ũ���� B. Ũ���� C. ����������Һ D. ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���������о��꼶�п�ģ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ�������
���л�ѧ����ʽ������������д��ȷ����
A. ������ˮ�����Ե�ԭ��CO2+H2O�TH2CO3
B. ͭ���������м��ȣ�Cu+O2 CuO2
CuO2
C. ʵ������ȡ������2H2O2 2H2O+O2��
2H2O+O2��
D. ֤�����������ᷴӦ��2Fe+6HCl�T2FeCl3+3H2��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com