
(1) �����£���֪0.1 mol��L��1һԪ��HA��Һ��c(OH��) / c(H+)��1��10��8��
�ٳ����£�0.1 mol��L��1 HA��Һ��pH= ��д�����ᣨHA����NaOH��Һ��Ӧ�����ӷ���ʽ�� ��
��pH��3��HA��pH��11��NaOH��Һ�������Ϻ���Һ��4���������ʵ���Ũ�ȴ�С��ϵ�ǣ� ��
(2) �����£���pH=a�İ�ˮ�м�����������ʱ����Һ�����ԣ���������pH 14��a��������������
(3) �����ʵ���Ũ�Ⱦ�Ϊ0.01mol��L-1��MnCl2��BaCl2�����Һ�У��μ�Na2CO3��Һ���ȳ����������� �����������ܵ���ʹ���ʱ����Һ��c(Ba2+)/c(Mn2+) �������¶��£�Ksp(BaCO3)=8.1��10��9��Ksp(MnCO3)=1.8��10��11��
��4����Ka��Kh��Kw�ֱ��ʾCH3COOH�ĵ���ƽ�ⳣ����CH3COO����ˮ��ƽ�ⳣ����ˮ�����ӻ�������������֮��Ĺ�ϵΪ��
��14�֣�
��1����3 �� HA+OH��=A��+H2O (��2��)
��c(A��)>c(Na+)>c(H+)>c(OH��) (2��)
��2���� (2��)
��3��Mn2+��450 ��2�֣�
��4��Ka��Kh��Kw (2��)
��������
�����������1����c��OH����/c��H����=1��10-8��c��OH������c��H����=1��10-14������c��OH����=10-11mol��L��1����c��H����=10-3mol��L��1��������Һ��pH=3��������Ũ��С�����Ũ�ȣ����Ը��������ᣬ���ᣨHA����NaOH��Һ��Ӧ�����ӷ���ʽΪ��HA+OH���TA��+H2O��
�ʴ�Ϊ��3��HA+OH���TA��+H2O��
��pH=11��NaOH��Һ��c��OH����=10-3mol��L��1��HA�����ᣬ���Ũ��ԶԶ����������Ũ�ȣ�����pH=3��HA��pH=11��NaOH��Һ�������Ϻ���Һ�е�����������Σ���Һ�����ԣ�������Һ��������Ũ�ȴ�������������Ũ�ȣ��������Ũ�ȴ���������Ũ�ȣ���Һ��4���������ʵ���Ũ�ȴ�С��ϵ��c��A������c��Na������c��H������c��OH������
�ʴ�Ϊ��c��A������c��Na������c��H������c��OH������
��2�����谱ˮ��ǿ�����ʱ��pH=a�İ�ˮ������������Ũ��=10a-14mol��L��1����pH=a�İ�ˮ�м�����������ʱ����Һ�����ԣ�������������Ũ�ȵ���������Ũ�ȣ����������pH=14-a��ʵ���ϰ�ˮ��������������ͼ��Ϻ���Һ�����ԣ�˵�����Ũ�ȴ��ڼ��Ũ�ȣ��������pH��14-a����ѡ����
��3��̼�ᱵ���ܶȻ���������̼���̵��ܶȻ������������������ȳ��������������ܵ���ʹ���ʱ����
c��CO32����=c��Mn2����= ����Һ��c��Ba2����=
����Һ��c��Ba2����=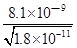 ��c��Ba2������c��Mn2����=
��c��Ba2������c��Mn2����=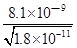 ��
�� ==450��
==450��
�ʴ�Ϊ��Mn2����450��
��4��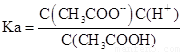 ��
��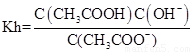 ��Kw=C��H������C��OH����������Ka��Kh=Kw��
��Kw=C��H������C��OH����������Ka��Kh=Kw��
�ʴ�Ϊ��Ka��Kh=Kw��
���㣺���������ˮ��Һ�еĵ���ƽ�⣻pH�ļ��㣻�����ʱ�Ķ����жϼ��й�ph�ļ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ���ữѧʽ | HClO | H2CO3 |
| ����ƽ�ⳣ�� | K=4.7��10-8 | K1=4.3��10-7��K2=5.6��10-11 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
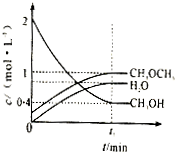 ��1������ˮú���ϳɶ����ѣ�CH3OCH3�����Ȼ�ѧ����ʽΪ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g������H=-274KJ/mol���÷�Ӧ��һ�������µ��ܱ������дﵽƽ���Ϊͬʱ��߷�Ӧ���ʺͶ����ѵIJ��ʣ����Բ�ȡ�Ĵ�ʩ��
��1������ˮú���ϳɶ����ѣ�CH3OCH3�����Ȼ�ѧ����ʽΪ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g������H=-274KJ/mol���÷�Ӧ��һ�������µ��ܱ������дﵽƽ���Ϊͬʱ��߷�Ӧ���ʺͶ����ѵIJ��ʣ����Բ�ȡ�Ĵ�ʩ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com