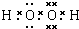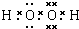MnO2����ѧ��ѧ�г�����һ���Լ��������������ʹ�����ij��ȤС��ͨ��ʵ���MnO2�����ʽ�����һϵ�е��о���
(1)��̽��MnO2�Ĵ�Ч������Ҫ��30%��H2O2��Һ���ܶȽ���Ϊ1 g/cm3������Ũ��Ϊ3%��H2O2��Һ ���ܶȽ���Ϊ1g/cm3��100 mL�����������Ʒ�����_________________________��
(2)��С�����������4����������֤MnO2�������ԣ����е���________��
A����MnO2������뵽FeSO4��Һ�У��ټ���KSCN��Һ���۲���Һ�Ƿ���
B����MnO2������뵽FeCl3��Һ�У��ټ���KSCN��Һ���۲���Һ�Ƿ���
C����MnO2������뵽Na2SO3��Һ�У��ټ���BaCl2��Һ���۲��Ƿ��а�ɫ��������
D����MnO2������뵽ϡ�����У��۲��Ƿ��л���ɫ��������
(3)��С��Ϊ�о��ڲ�ͬ����Ե���Һ��MnO2���������������ǿ���KI��Һ��Ũ�Ⱥ�MnO2�����������ͬ���㶨ʵ���¶���298K��������¶Ա�ʵ�顣
�������Ա�ʵ���У����Եó��Ľ�����__________________��д�������������£�MnO2����I-�����ӷ���ʽ_____________________��
(4)���ö������̵������ԣ�������Ũ���ᷴӦ��ȡ������������������Ϊ�÷�Ӧ�ķ�Ӧ��������
_________ ������ţ���




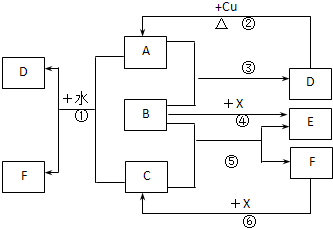
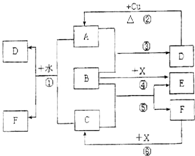 ��֪A��F����ѧ��ѧ�г������ʣ�����A��C��EΪ���壬B��DΪҺ�壬D��һ�ֲ��ӷ����ᣬ��Ũ��Һ��ǿ�����ԣ�F����Һ��X����ͨ������ʵ�����Ʊ�����C��X��һ�ֺ�ɫ��ĩ��B��������18�����ӣ�ʵ���ҳ���B��X�Ʊ�����E����Ӧ�в�������������ȥ���Իش��������⣺
��֪A��F����ѧ��ѧ�г������ʣ�����A��C��EΪ���壬B��DΪҺ�壬D��һ�ֲ��ӷ����ᣬ��Ũ��Һ��ǿ�����ԣ�F����Һ��X����ͨ������ʵ�����Ʊ�����C��X��һ�ֺ�ɫ��ĩ��B��������18�����ӣ�ʵ���ҳ���B��X�Ʊ�����E����Ӧ�в�������������ȥ���Իش��������⣺