
(6��)(1)ijѧ�������к��Ȳⶨ��ȡ��50 mL 0��50 mol��L��1��HCl��50 mL 0��55 mol��L��1 ��NaOH��Һ(�ܶȶ���1 g��cm��3)��ʵ���õ��������ݣ�

(�кͺ����ɵ���Һ�ı�����Ϊ4��18J��g��1������1)�����ͬѧ������к��ȵ�ƽ��ֵ����������������������������������������������������������
(2)��ѧ����õ����ݱ�����ֵ��������(��ߡ��͡�)��
(3)�����з�����ѡ����ѧ������ʵ������ԭ�������(��д��ĸ)����������
A����Һ��Ϻ�δ��ʱ�Ǻ����ȼƱ���
B���㵹��Һ̫�죬�����������ձ�
C����Һ��Ϻ���費��
D��δ���¶��������ֵ�ͼ�¼�¶ȼ�ʾ��
E������Ͳ��ȡ�������ʱ���Ӷ���
F���ձ��Ͳ�����������һ��������
 ����������������ϵ�д�
����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g/cm3�� |
| �Ҵ� | -117.0 | 78.0 | 0.79 |
| ���� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���ᣨ98%�� | - | 338.0 | 1.84 |
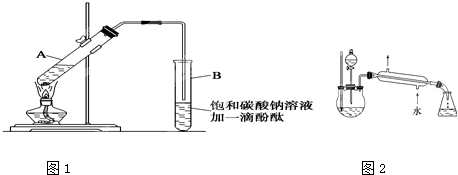
| Ũ���� |
| ���� |
| Ũ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����У�Ϻ��У�����������ʦ������У�����������ѧ�Ծ����������� ���ͣ�������
�����6�֣�
ijѧ��������ȤС��Ϊ�˲ⶨþ���Ͻ������ĺ���������������ʵ�顣���Ͻ�3.0��Ͷ�뵽������100 mL 1.5 mol?L-1���ռ���Һ�У���ַ�Ӧ������δ��Ӧ��þ��Ȼ������Һ�еμ�1.0 mol?L-1�����ᣬ��������������õ��������������±���
| ʵ����� | ������������ | �������� |
| 1 | 60 mL | 0 |
| 2 | 80 mL | 0.78 g |
| 3 | 180 mL | 5.46 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ��ƽһ�߸߶���һ���¿���ѧ�Ծ����������� ���ͣ�ʵ����
(6��)(1)ijѧ�������к��Ȳⶨ��ȡ��50 mL 0��50 mol��L��1��HCl��50 mL 0��55 mol��L��1��NaOH��Һ(�ܶȶ���1 g��cm��3)��ʵ���õ��������ݣ�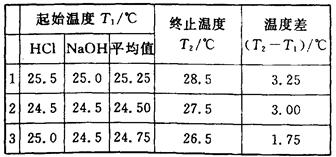
(�кͺ����ɵ���Һ�ı�����Ϊ4��18J��g��1������1)�����ͬѧ������к��ȵ�ƽ��ֵ����������������������������������������������������������
(2)��ѧ����õ����ݱ�����ֵ��������(��ߡ��͡�)��
(3)�����з�����ѡ����ѧ������ʵ������ԭ�������(��д��ĸ)����������
| A����Һ��Ϻ�δ��ʱ�Ǻ����ȼƱ��� |
| B���㵹��Һ̫�죬�����������ձ� |
| C����Һ��Ϻ���費�� |
| D��δ���¶��������ֵ�ͼ�¼�¶ȼ�ʾ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ����У�Ϻ��У�����������ѧ�Ծ��������棩 ���ͣ�������
�����6�֣�
ijѧ��������ȤС��Ϊ�˲ⶨþ���Ͻ������ĺ���������������ʵ�顣���Ͻ�3.0��Ͷ�뵽������100 mL 1.5 mol•L-1���ռ���Һ�У���ַ�Ӧ������δ��Ӧ��þ��Ȼ������Һ�еμ�1.0 mol•L-1�����ᣬ��������������õ��������������±���
|
ʵ����� |
������������ |
�������� |
|
1 |
60 mL |
0 |
|
2 |
80 mL |
0.78 g |
|
3 |
180 mL |
5.46 g |
��1����ʼ����ʱ������������������� mL�����õ������������ʱ��������������Ϊ mL��
��2���Ͻ���������������Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com