
2016��ŵ������ѧ�������ڡ����ӻ�����ƺͺϳɡ�������ͻ���ɾ͵���λ��ѧ�ң����о�����֮һ�����ӿ��ء������������ӱ������� ����ӻ����������йء��ش��������⣺
(1)���嶡����[4]��������ͼ��ʾ�������ڢ�B��Ԫ�ض�Ӧ��������ȡ����La2+��Sc2+��д����̬���������ӣ�Sc2+����������Ų�ʽ��__________�����е���ռ�ݵĹ����Ϊ__________����

(2)���嶡����[4]������4���������ɱ��ף�����������ԭ�ӵ��ӻ���ʽΪ___________����������������Ϊ_______________��
(3)��ͬ��С�ı�������ʶ��ijЩ���ӣ��磺N3-��SCN-�ȡ����ݵȵ�����ԭ���ж�N3-�ռ乹��Ϊ______��һ�������£�SCN-��MnO2��Ӧ�ɵõ�(SCN)2����д��(SCN)2 �Ľṹʽ��_______________��
(4)��֪C60���ӽṹ��C60����ʾ��ͼ����ͼ��ͼ����ʾ����
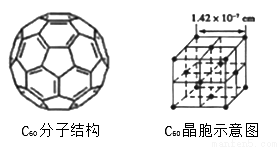
��һ��C60�����к��ЦҼ�����Ϊ__________����ÿ��C60���Ӿ����������ȵ�C60������_________����C60������ܶ�Ϊ__________��������������λС������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ��ɽ�и�һ3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���з����У�����ԭ�Ӷ����������8���ӽṹ���ǣ�
A. ���� B. �������� C. ���Ȼ��� D. ����̼
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ӱ�ʡ��ɽ��2016-2017ѧ��߶�3���¿���ѧ�Ծ� ���ͣ������
ij�������Ļ�����A������Է�������Ϊ104��̼����������Ϊ92.3%��
(1)A�ķ���ʽΪ____________��
(2)A��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽΪ________________________��
��Ӧ������____________��
(3)��֪��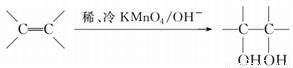 ����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ��������Ľṹ��ʽ_______________��
����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ��������Ľṹ��ʽ_______________��
(4)��A��Ϊͬ���칹���һ������Ϊ������(����ͼ)����������Ķ��ȴ�����__________________�֡�
(5)��һ�������£���A�ۺϵõ��ĸ߷��ӻ�����Ļ�ѧ����ʽΪ_________________________________________����Ӧ����Ϊ_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ӱ�ʡ��ɽ��2016-2017ѧ��߶�3���¿���ѧ�Ծ� ���ͣ�ѡ����
���з�Ӧ������ȡ����Ӧ����
A. ��ϩʹ���Ը��������Һ��ɫ
B. ��ϩ��������Ȼ�̼��Һ��Ӧ
C. ����Ũ�����Ũ����Ļ��Һ���ȷ�Ӧ
D. �ڱ��е�����ˮ����ˮ�����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ӱ�ʡ��ɽ��2016-2017ѧ��߶�3���¿���ѧ�Ծ� ���ͣ�ѡ����
����һ��ȡ����ֻ���������ַе㲻ͬ�����������
A����CH3��2CHCH2CH2CH3 B����CH3��3CCH2CH3
C����CH3��2CHCH��CH3��2 D����CH3��2CHCH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�찲��ʡ�Ϸ��и����ڶ��ν�ѧ����������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����������Խ��Al-Ag2O2������ڵ������[CO(NH2)2]�ļ�����Һ�Ʊ���������Ĥ�����ֹ����ͨ����a��b��Ϊ���Ե缫��������˵����ȷ����
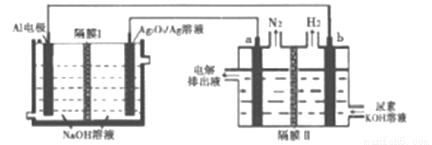
A. Ag�缫����������Ӧ��õ缫����ҺpH��С
B. ԭ��ص��ܷ�ӦΪ��2Al+3Ag2O2+2NaOH=2NaAlO2+3Ag2O+H2O
C. a�缫�ϵĵ缫��ӦΪ��CO(NH2)2+8OH--6e-=CO32-+N2��+6H2O
D. ÿ����2.7g����������a��b��������������3.36L(��״��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�츣��ʡ������ѧ��ģ��4�����ۺϻ�ѧ�Ծ��������棩 ���ͣ������
ͭ��Ӧ�ý�Ϊ�㷺����ɫ������
��1����̬ͭԭ�ӵļ۵����Ų�ʽΪ_____________��
��2������������Cu2Zn�Ͻ���нϸߵ��۵㡢�ϴ��ǿ�ȡ�Ӳ�Ⱥ���ĥ�ȡ���Cu2Zn�Ͻ�ľ���������______��
��3��ij��ͭ����������ӽṹ��ͼ��ʾ��

�� �������д��ڵ���������__________��
a�����Ӽ� b�����ۼ� c����λ��
d����� e�����»���
�� �������еڶ����ڵķǽ���Ԫ�صĵ�һ�������ɴ�С��˳����______��
�� ��������Nԭ�ӵ��ӻ�������_________��
��4����������������Ҫ�أ�
�� ԭ�������������ʾ�����ڲ���ԭ�ӵ����λ�ã���ͼΪͭ�����γɵ�ij�����ᄃ��������ԭ���������A Ϊ��0��0��0����BΪ�� ��0��
��0�� ����C��
����C�� ��
�� ��0������Dԭ�ӵ��������Ϊ_____________��
��0������Dԭ�ӵ��������Ϊ_____________��
�� �������������������Ĵ�С����״���辧���ı߳�Ϊapm����O����λ����_______��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�걱���л�������һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪��Ӧ����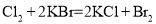 ����
����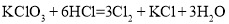 ����
����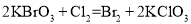 ������˵����ȷ����
������˵����ȷ����
A. ����������Ӧ���е������ɣ����Զ����û���Ӧ
B. ��������ǿ����˳��Ϊ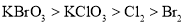
C. ��Ӧ���л�ԭ���������������ʵ���֮��Ϊ6:1
D. ����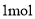 ��ԭ����Ӧ���������õ����ӵ����ʵ���Ϊ
��ԭ����Ӧ���������õ����ӵ����ʵ���Ϊ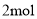
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������л�����2016-2017ѧ��ȵ�һѧ����ĩ���Ը߶���ѧ�Ծ� ���ͣ�ѡ����
�����г��������漰��ѧ֪ʶ��ijЩ���⣬����������ȷ���ǣ� ��
���˵�Ƥ����ǿ�����ߵ������½���ʧȥ��������
���ü�ȩ��Һ���ݺ���Ʒ����
�۱��ʵ���֬�����ŵ�������ζ����������֬������ˮ�ⷴӦ
�ܳ����õ���ʳ�Ρ����Ǿ����Է���
A. �٢� B. �ڢ� C. �٢ڢ� D. ȫ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com