


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�� ��� |
HA���ʵ��� Ũ��/��mol?L-1�� |
NaOH���ʵ��� Ũ��/��mol?L-1�� |
��Ϻ��� Һ��pH |
| �� | 0.1 | 0.1 | pH=a |
| �� | 0.12 | 0.1 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���������һ�и�����ѧ�ڵڶ����¿������ۣ���ѧ ���ͣ�ʵ����
��18�֣�ʵ��һ��ij��ѧС��Ϊ�Ƚ�����ʹ�������ԣ����������ʵ�鷽����װ����ͼ��
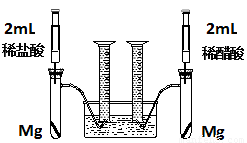
ʵ�鷽���������Թ��зֱ�������þ����ͬʱ����ע�����е���Һע����Ӧ�Թ��У��۲�������������ʺ������
��1��������þ��Ӧ�����ӷ���ʽΪ ��
��2��������ʵ�鷽������һ����Ƿȱ����Ƿȱ�� ��
��3����Ƿȱ�Ѿ������ķ����£���Ӧ��ʼʱ���������������ʹ�ϵӦ�� �����ղ��������������ϵӦ�� ��
��4��ʵ���в������������������ֵ�ߣ�����ԭ���� ��
��5��ͨ���Ƚ���ʼ��Ӧ�����ʿ��Եó��Ľ����� ��
��6�������������⣬������ͨ�����������Ƚ�����ʹ�������ԣ���д������һ�ָ��ķ���
��
ʵ�����ij��Ԫ�ᣨH2B����ˮ�еĵ��뷽��ʽ�ǣ� H2B=H++HB��
HB�� H++B2��
H++B2��
�ش��������⣺
��1�����ж�H2B��ǿ����ʻ���������ʣ�
��2����֪0.1mol��L��1 NaHB��Һ��pH=2����0.1mol��L-1 H2B��Һ�������ӵ����ʵ���Ũ��Ӧ 0.11 mol��L��1���������������������
��3����0.1mol/L��Na2B��Һ�У���������Ũ�ȹ�ϵʽ��ȷ����_______��
A��c(H+)+c(HB-)+c(H2B)=0.1mol/L B��c(Na+)+c(OH-)=c(H+)+ c(HB-)
C��c(Na+)+ c(H+)= c(OH-)+ c(HB-)+2c(B2-) D��c(Na+)=2c(B2-)+2 c(HB-)
��4��0.1mol/LNaHB��Һ�и�������Ũ���ɴ�С��˳����_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ��ˮһ�и߶����ϣ����л�ѧ�Ծ������ƣ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꼪��ʵ����ѧ�߶����ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com