
����Ŀ��ʵ������Ҫ����0.5molL-1���ռ���Һ480mL��������Һ���ƵĹ��̣��ش��������⣺
��1��ʵ���г���������ƽ�������룩��ҩ�ס��ձ�����Ͳ�Ͳ������⣬����Ҫ���������������У�___��___��
��2�����ݼ����֪������NaOH���������Ϊ___g��
��3��ʵ�鿪ʼǰ����Ҫ��___��
��4��������Һ�Ĺ����У������²�����������ȷ����___������ţ���
A�����������ƹ������ֽƬ�ϳ�����
B�����ձ����ܽ��������ƹ������������Һ��������ƿ�У�
C�����ܽ��������Ƶ��ձ�������ˮϴ��2��3�Σ�����ϴ��Һת�Ƶ�����ƿ��
��5�����и�������У����ܵ���ʵ��Ũ��ƫ�ߵ���___����ѡ����ĸ��
A������NaOH����ʱ����������ʵ�λ�öԵ�
B��������ƿ��ת��ʱ����������
C��NaOH�ܽ�ʱ�ų��������ȣ�δ����ȴ����������Һ
D������ʱ����������ƿ�Ŀ̶���
E������ʱ����ˮ�����̶��ߣ����ý�ͷ�ι���������IJ���
���𰸡�500mL����ƿ ��ͷ�ι� 10.0 ��© C C
��������
��1����������һ�����ʵ���Ũ�ȵ���Һ����ѡ��������Ȼ���жϻ�ȱ�ٵ�������
��2��ʵ����û��480mL������ƿ����Ҫѡ��500mL������ƿ������m=nM�����500mL 0.5mol/L������������Һ�к����������Ƶ�������
��3������ƿ��Ҫ��©��
��4��������������������Һ����ȷ�������������жϣ�
��5���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=![]() �����жϡ�
�����жϡ�
��1��û�й��Ϊ480mL������ƿ������ʱ��Ҫѡ��500mL������ƿ��ʵ�������Ƶ���500mL 0.5mol/L������������Һ�����Ʋ���Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ���Ҫʹ�õ������У�������ƽ�����������ձ���ҩ�ס�500mL����ƿ����ͷ�ιܵȣ���ȱ�ٵIJ�������Ϊ��500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��2������500mL 0.5mol/L������������Һ����Ҫ�������Ƶ�����Ϊ��m(NaOH)=40g/mol��0.5mol/L��0.5L=10.0 g��
�ʴ�Ϊ��10.0��
��3��ʵ�鿪ʼǰ����Ҫ�ȼ�©���ʴ�Ϊ����©��
��4��A���������ƾ��н�ǿ��ʴ�ԡ��׳��⣬Ӧ�÷����ձ��г�����A�����
B���������ƹ������ܽ�����л�ų�������Ӧ����ȴ����ת�Ƶ�����ƿ�У�����ᵼ�����Ƶ���ҺŨ��ƫ�ߣ�B�����
C���ձ��Ͳ������ϻ�ճ�в������ʣ��������ƹ�����Ӧ�ý��ܽ��������Ƶ��ձ�������ˮϴ��2��3�Σ�����ϴ��Һת�Ƶ�����ƿ�У�C����ȷ��
�ʴ�ΪC��
��5��A������NaOH����ʱ����������ʵ�λ�öԵ�����δʹ�����룬���������Ƶ�������Ӱ�죬��������ҺŨ����Ӱ�죬��ʹ�����룬�������Ƶ�ʵ��������С��������Һ��Ũ��ƫ�ͣ����������⣬A�����
B��������ƿ��ת��ʱ������������������������ƿ���������Ƶ�������С��������ҺŨ��ƫ�ͣ�B�����
C��Һ������������������ʣ����������ܽ���ȣ�δ��ȴ�����£����Ƚ���Һ��������ƿ�����������Һ���ᵼ����Һ���ƫС����ҺŨ��ƫ�ߣ�C����ȷ��
D������ʱ����������ƿ�̶��ߣ�ʹ��Һ�����ƫ��������ҺŨ��ƫ�ͣ�D�����
E������ʱ����ˮ�����̶��ߣ�ʹ��Һ�����ƫ��������ҺŨ��ƫ�ͣ���Һ�Ǿ��ȵģ����ý�ͷ�ι���������IJ��֣�ʣ����Һ��������ҺŨ����ͬ��E�����
�ʴ�Ϊ��C��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͭ�����ճ������г����Ľ��������Ź㷺����;����ش��������⣺
(1)�����Fe(CO)x�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe(CO)x��������__________(�������)��Fe(CO)x������ԭ�Ӽ۵������������ṩ������֮��Ϊ18����x=_____________��![]() �ĺ�������Ų�ʽΪ_____________________��
�ĺ�������Ų�ʽΪ_____________________��
(2)![]() ��Һ�����ڼ���_________(�����ӷ���)��
��Һ�����ڼ���_________(�����ӷ���)��![]() ��̼ԭ���ӻ��������Ϊ_____��1mol
��̼ԭ���ӻ��������Ϊ_____��1mol![]() ���еĦм���ĿΪ_______(��N��ʾ)��C��N��O��һ�������ɴ�С��˳��Ϊ_________(��Ԫ�ط��ű�ʾ)��
���еĦм���ĿΪ_______(��N��ʾ)��C��N��O��һ�������ɴ�С��˳��Ϊ_________(��Ԫ�ط��ű�ʾ)��
(3)ijMԭ�ӵ���Χ�����Ų�ʽΪ![]() ��ͭ��M�γɵ�ij������ľ����ṹ����ͼ��ʾ(�ڵ����ͭԭ��)��
��ͭ��M�γɵ�ij������ľ����ṹ����ͼ��ʾ(�ڵ����ͭԭ��)��

�ٸþ���Ļ�ѧʽΪ__________________��
����֪ͭ��M�ĵ縺�Էֱ�Ϊ1.9��3.0����ͭ��M�γɵĻ���������___________(����ӡ����ۡ�)�����
����֪�þ�����ܶ�Ϊ![]() �������ӵ�����Ϊ
�������ӵ�����Ϊ![]() ����þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���Ϊ____________________pm(ֻ��д������ʽ)��
����þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���Ϊ____________________pm(ֻ��д������ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ɽ����������ȡ��һ�־������Կ������Ե��л���X����ṹ��ʽ����ͼ��ʾ�������й�˵���������

A. �����ʵķ���ʽΪC10H16O2
B. �����ʲ��������е�̼ԭ�ӹ�ƽ��
C. �����ʵ�һ�ȴ��ﹲ��7��
D. �������ܷ����ӳɡ�ȡ������������ȥ����ԭ�ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��C60�����ʯ��ʯī�Ľṹģ����ͼ��ʾ(ʯī����ʾ�����е�һ��ṹ)��


(1)C60�����ʯ��ʯī���ߵĹ�ϵ�ǻ�Ϊ________��
A��ͬ���칹�� B��ͬ�������� C��ͬϵ�� D��ͬλ��
(2)��̬ʱ��C60����___(����ӡ�����ԭ�ӡ����ӡ�)���壬C60�����к���˫������Ŀ��___��
(3)�辧��Ľṹ�����ʯ���ƣ�1 mol�辧���к��й�赥������ĿԼ��______NA����
(4)ʯī��״�ṹ�У�ƽ��ÿ����������ռ�е�̼ԭ������________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ������ˮ��Һ�����ܺ������������е������֣�K+��NH4+��Ba2+��CO32-��Cl-��SO42-����ȡ����200mL��Һ�ֱ��������ʵ�飺
�ٵ�һ�ݼ�����NaOH��Һ�����ȣ��ռ�������1.36g��
�ڵڶ��ݼ�����BaCl2��Һ�ø������12.54g������������ϴ�ӡ������������Ϊ4.66g��
��������ʵ�飬�����Ʋ���ȷ���ǣ� ��
A.һ������NH4+��CO32-��SO42-��һ��������Ba2+��Cl-
B.һ������NH4+��CO32-��Cl-��SO42-����������K+
C.c(SO42-)=0.2mol/L��c(NH4+)��c(SO42-)
D.����Һ�д���K+��NH4+��CO32-��Cl-��SO42-�������ӣ���c(K+)��0.2mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦA2+B2![]() 2AB�ڲ�ͬ�����£�����AB�ٷֺ�����ʱ��仯��ϵ��ͼ��ʾ��aΪ500����bΪ300��ʱ�����cΪ300��ʱ��ʱ��t3��ʼ�������м�ѹ�������������������ȷ����
2AB�ڲ�ͬ�����£�����AB�ٷֺ�����ʱ��仯��ϵ��ͼ��ʾ��aΪ500����bΪ300��ʱ�����cΪ300��ʱ��ʱ��t3��ʼ�������м�ѹ�������������������ȷ����

A. A2��B2��AB��Ϊ���壬����Ӧ����
B. ABΪ���壬A2��B2��������һ��Ϊ�����壬����Ӧ����
C. ABΪ���壬A2��B2��������һ��Ϊ�����壬����Ӧ����
D. ABΪ���壬A2��B2��������һ��Ϊ�����壬����Ӧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
�Է�����AΪԭ���Ʊ�ij��Ҫҽҩ�м���F�ĺϳ�·�����£�
![]()
�Իش��������⣺
(1)B�Ļ�ѧ����Ϊ___________��
(2)F�����������ŵ�����Ϊ___________��
(3)��A����B����D����E�ķ�Ӧ���ͷֱ���___________��___________��
(4)��֪G�ķ���ʽΪC4H9Br2N����һ��������C��G��Ӧ���� ��д���÷�Ӧ�Ļ�ѧ����ʽ___________��
��д���÷�Ӧ�Ļ�ѧ����ʽ___________��
(5)д��ͬʱ��������������D������ͬ���칹��Ľṹ��ʽ___________��___________��
�ٺ��б������ҷ����к���4�ֲ�ͬ��ѧ�������⣻
�ڼ��������ᷴӦ�����ܷ���������Ӧ��
(6)����![]() ��(CH3)2SO4��CH3CH2OHΪԭ�ϣ���ϱ�����Ϣ������ͼ�е�ͼ����д���Ʊ�
��(CH3)2SO4��CH3CH2OHΪԭ�ϣ���ϱ�����Ϣ������ͼ�е�ͼ����д���Ʊ�![]() �ĺϳ�·������ͼ(�������Լ���ѡ)��
�ĺϳ�·������ͼ(�������Լ���ѡ)��
_______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������Ҫ���л��ϳ��м��壬��һ�ֺϳ�·�����£�
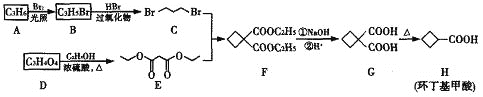
��ش��������⣺
(1)����������ķ���ʽΪ____________________��
(2)������������ԭ��A��D��һϵ�з�Ӧ�Ƶã�AΪϩ������A������Ϊ______��D���ʵĹ�����Ϊ_______��
(3)д��D��E�Ļ�ѧ����ʽ________________________��
(4)C+E��F�ķ�Ӧ����Ϊ_________________________��
(5)������WΪH��ͬ���칹�壬�ܷ���������Ӧ��ֻ������һ�ֹ����ţ������з���������W�Ľṹ��ʽΪ_____________��
(6)���������ϳ�·�ߣ��� ![]() ��EΪԭ��(���Լ���ѡ)������Ʊ�
��EΪԭ��(���Լ���ѡ)������Ʊ�![]() �ĺϳ�·�ߣ�__________��
�ĺϳ�·�ߣ�__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʱ��1mol/L��HA��1mol/L��HB��������Һ����ʼʱ�������ΪV0���ֱ�������Һ�м�ˮ����ϡ�ͣ����ñ仯��ϵ��ͼ��ʾ��V��ʾ��Һϡ�ͺ�������������˵���������

A. Ka( HA)ԼΪ10-4
B. ������Һ��ϡ����![]() ʱ����Һ��
ʱ����Һ��![]() ��
��![]()
C. �к͵����pH��ͬ������������n(NaOH)��HA>HB
D. ������������ʵ���Ũ�ȵ�NaA��NaB��Һ����������ǰ��С�ں���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com