
ijͬѧΪ��̽���Ȼ�淋����ʣ�����������ʵ�飬���㰴Ҫ��ش��������⡣
��1������100mL1mol/L��NH4Cl��Һ����ͬѧӦ��������ƽ����NH4Cl���������Ϊ g��
�������������ձ�����ͷ�ιܡ��������Ȳ���������
 �ٻ�ȱ�ٵ������� ��
�ٻ�ȱ�ٵ������� ��
��ʹ������ƿǰ������е�һ�������� ��
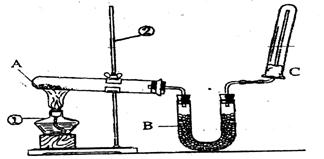
��2����ͬѧ������ͼ��ʾ��װ�������йذ���������ʵ�顣
��д��ʵ�����ư����Ļ�ѧ����ʽ�� ��
��д��B��ʢ�ŵ��Ǽ�ʯ���������� ��
�۰�����������ˮ���ڰ�ˮ�еμӷ�̪������ ��
�ܼ��鰱���ķ����� ��
��6�֣���ϡ�����з���������ͭƬ��
��1����Ӧ�Ļ�ѧ����ʽΪ ��
��2������Ӧֹͣ���ټ�������25%��ϡ���ᣬ��ʱͭƬ���������ݲ�������ԭ���� ���������ӷ���ʽ��ʾ��
��3������12.8gͭ��һ������Ũ���ᷴӦ��ͭ������ʱ������������5.6L����
״���£����������ĵ���������ʵ����� ��
�� ��8�֣�
��1��5.4g����100mL����ƿ �ڼ���Ƿ�©ˮ
��2���٢�Ca(OH)2 + NH4Cl ![]() CaCl2 + 2NH3�� + 2H2O
CaCl2 + 2NH3�� + 2H2O
������ˮ���� ����Һ����ɫ��Ϊ��ɫ
����ʪ��ĺ�ɫʯ����ֽ ��պ��Ũ����IJ��������������ɸ���)
��6�֣�ÿ��2�֣�Ks5u
��1��3Cu + 8HNO3��ϡ��= 3Cu(NO3)2 + 2NO�� + 4H2O
��2��3Cu + 8H+ + 2NO3-= 3Cu2+ + 2NO�� + 4H2O
��3��0.65mol
Ks5u



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��ɽ��ʡɽ����2011��2012ѧ��߶�5���¿���ѧ���� ���ͣ�058
��ijͬѧΪ��̽���Ȼ�淋����ʣ�����������ʵ�飬���㰴Ҫ��ش��������⣮
(1)����100 mL��1 mol/L��NH4Cl��Һ����ͬѧӦ��������ƽ����NH4Cl���������Ϊ________g��
�������������ձ�����ͷ�ιܡ��������Ȳ���������
�ٻ�ȱ�ٵ�������________��
��ʹ������ƿǰ������е�һ��������________��
(2)��ͬѧ������ͼ��ʾ��װ�������йذ���������ʵ�飮
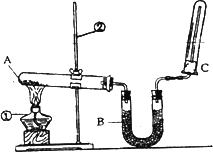
��д��ʵ�����ư����Ļ�ѧ����ʽ��________��
��д��B��ʢ�ŵ��Ǽ�ʯ����������________��
�۰�����������ˮ���ڰ�ˮ�еμӷ�̪������________��
�ܼ��鰱���ķ�����________��
����ϡ�����з���������ͭƬ��
(1)��Ӧ�Ļ�ѧ����ʽΪ________��
(2)����Ӧֹͣ���ټ�������25����ϡ���ᣬ��ʱͭƬ���������ݲ�������ԭ����________��(�����ӷ���ʽ��ʾ)
(3)����12.8 gͭ��һ������Ũ���ᷴӦ��ͭ������ʱ������������5.6 L(��״����)���������ĵ���������ʵ�����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡɽ���и߶�5���¿���ѧ�Ծ����������� ���ͣ�ʵ����
��8�֣�ijͬѧΪ��̽���Ȼ�淋����ʣ�����������ʵ�飬���㰴Ҫ��ش��������⡣
��1������100mL1mol/L��NH4Cl��Һ����ͬѧӦ��������ƽ����NH4Cl���������Ϊ g��
�������������ձ�����ͷ�ιܡ��������Ȳ���������
�ٻ�ȱ�ٵ������� ��
��ʹ������ƿǰ������е�һ�������� ��
��2����ͬѧ������ͼ��ʾ��װ�������йذ���������ʵ�顣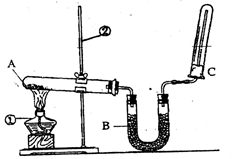
��д��ʵ�����ư����Ļ�ѧ����ʽ�� ��
��д��B��ʢ�ŵ��Ǽ�ʯ���������� ��
�۰�����������ˮ���ڰ�ˮ�еμӷ�̪������ ��
�ܼ��鰱���ķ����� ��
��6�֣���ϡ�����з���������ͭƬ��
��1����Ӧ�Ļ�ѧ����ʽΪ ��
��2������Ӧֹͣ���ټ�������25%��ϡ���ᣬ��ʱͭƬ���������ݲ�������ԭ���� ���������ӷ���ʽ��ʾ��
��3������12.8gͭ��һ������Ũ���ᷴӦ��ͭ������ʱ������������5.6L����
״���£����������ĵ���������ʵ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������˫ʮ��ѧ������ǰ�����������ۺϣ���ѧ�� ���ͣ������
��15�֣���ij�о���ѧϰС��Թ���̿������������Ӧ���������ɷֽ����о���
��1��������裺�ٸ÷�Ӧ�����������CO2���ڸ÷�Ӧ�����������CO���۸÷�Ӧ����������� 
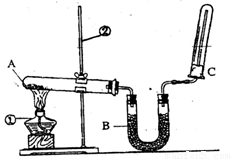
��2����Ʒ�������ͼ��ʾ����һ�������������ڸ��������������������̿����ȫ��Ӧ���ⶨ�μӷ�Ӧ��̼Ԫ������Ԫ�ص������ȡ�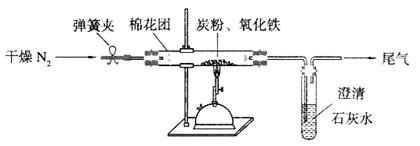
��3���������ϣ���������̼��������������Ӧ��ʵ���ҿ������Ȼ�隣�����Һ���������ƣ�NaNO2��������Һ��ϼ��ȷ�Ӧ�Ƶõ�����
����д���÷�Ӧ�����ӷ���ʽ�� ��
������װ�ÿ�����ʵ������ȡ�������� ��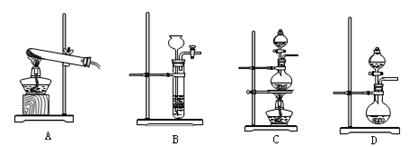
��4��ʵ�������ʵ������
�ٰ���ͼ����װ�ã������װ�õ������ԣ���ȡ3��20g��������2��00g̿�ۻ�Ͼ��ȣ�����48��48g��Ӳ�ʲ������У�
�ڼ���ǰ����ͨһ��ʱ�䴿������ĵ�����
��ֹͣͨ��N2�н����ɼУ�����һ��ʱ�䣬����ʯ��ˮ����ǣ�
�ܴ���Ӧ��������ͨһ��ʱ��ĵ�������ȴ�����£��Ƶ�Ӳ�ʲ����ܺ���������Ϊ52��24g��
��5�����ݴ����������㣬�μӷ�Ӧ��̼Ԫ������Ϊ0��48g����Ԫ��Ϊ0��96g���ƶϼ��� ������ ��ʵ���з����Ļ�ѧ����ʽΪ ��
��ʵ���з����Ļ�ѧ����ʽΪ ��
��6��ʵ���Ż�������ʵ��ó��Ľ��ۣ�Ӧ�Ը�ʵ��װ�ý�һ�����ƣ�����ΪӦ����θĽ��� ��
����̼��������ֱ�Ӻϳɼ״����Ҵ�ȼ���ѽ��빤ҵ�������磺
��Ӧ�� CO(g)+2H2(g)  CH3OH(g)
CH3OH(g)
��Ӧ�� 2CO(g)+4H2 (g) CH3CH2OH (g)+H2O (g)
CH3CH2OH (g)+H2O (g)
ijͬѧΪ��Ѱ�Һϳɼ״�����������[�¶ȡ�ѹǿ��̼���n(CO)/n(H2)����������]����������¶Ա�ʵ�飬����ʵ�������Ѿ���������ʵ����Ʊ��У�ÿ��ʵ�飬��Ӧʱ����ͬ����
| ʵ���� | T(��) | n (CO)/n(H2) | P(MPa) | COת����(%) |
| 1 | 150 | 1/3 | 0��1 | K^S*5U.C# |
| 2 | x | 1/3 | 5 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�߶�5���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
��8�֣�ijͬѧΪ��̽���Ȼ�淋����ʣ�����������ʵ�飬���㰴Ҫ��ش��������⡣
��1������100mL1mol/L��NH4Cl��Һ����ͬѧӦ��������ƽ����NH4Cl���������Ϊ g��
�������������ձ�����ͷ�ιܡ��������Ȳ���������
�ٻ�ȱ�ٵ������� ��
��ʹ������ƿǰ������е�һ�������� ��
��2����ͬѧ������ͼ��ʾ��װ�������йذ���������ʵ�顣
��д��ʵ�����ư����Ļ�ѧ����ʽ�� ��
��д��B��ʢ�ŵ��Ǽ�ʯ���������� ��
�۰�����������ˮ���ڰ�ˮ�еμӷ�̪������ ��
�ܼ��鰱���ķ����� ��
��6�֣���ϡ�����з���������ͭƬ��
��1����Ӧ�Ļ�ѧ����ʽΪ ��
��2������Ӧֹͣ���ټ�������25%��ϡ���ᣬ��ʱͭƬ���������ݲ�������ԭ���� ���������ӷ���ʽ��ʾ��
��3������12.8gͭ��һ������Ũ���ᷴӦ��ͭ������ʱ������������5.6L����
״���£����������ĵ���������ʵ����� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com