
����ͼ������ƿ�а��٢ڢۢܵ�˳�����μ����Լ��������뿪�����Թ۲쵽����������ɫ��ĭ����״�����ƿ��Ȼ���а�ɫ��ĭѸ�ٴ���ƿ���ݳ����������������д���ˮ����ð�������ִ�����ƿ���о��Ƚ��̣�
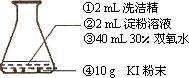
(1)��ʼ�������ĭ����ɫ��˵��������Ӧ�������˵��ʵ⣮��д��KI��H2O2��Ӧ���ɵ�����ӷ���ʽ________________________
(2)��֪1 molҺ̬H2O2�ֽ�����Һ̬ˮ���������ų�98.4 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ________________��
(3)����ʵ����KI��ĩ��KMnO4��ĩ���棬Ҳ�ɲ�������������H2O2�ֱ������������ʷ�Ӧʱ�����ֵ�������ʲô��ͬ��
________________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

������Ũ�Ⱦ�Ϊ1 mol��L-1������ʹ�����Һ��
����_________________��ȡ10.00 mL 1 mol��L-1����ʹ�����Һ�ֱ����������ƿ�У�
�۷ֱ��ȡ��ȥ��������Ĥ��þ��a g����ϵ��ͭ˿ĩ�ˣ�a����ֵ����Ϊ_____________��
���ڹ��ƿ��װ������ˮ������ͼ���Ӻ�װ�ã����װ�õ������ԣ�
�ݽ�ͭ˿�����ƶ���ʹ����þ����������(ͭ˿������Ӵ�)������Ӧ��ȫ����¼
__________________________________________________________________��
��Ӧ��������¶Ȼָ������£�������Һ���������Һ�棬��ȡ��Ͳ��ˮ�����ǰ��Ӧ______________________________________________��������Ͳ��ˮ�����ΪV mL��
�뽫�������貹������������������⣺
(1)�����ֱ����ܼ��װ�������ԵIJ�����۲췽����_______________________________��
(2)��ʵ����Ӧѡ��_________________ (�����)����Ͳ��
A.100 mL B.200 mL C.500 mL
(3)��ˮ������Ӱ����Բ��ƣ���ʵ���������£�����Ħ������ļ���ʽΪ��
Vm=_________________��
(4)�������ʲ��ȵ�ԭ����___________________________________________________��
ͭ˿������Ӵ���ԭ����___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

������Ũ�Ⱦ�Ϊ1 mol��L-1������ʹ�����Һ��
���ڹ��ƿ����װˮ����ͼʾ���Ӻ�װ�ã����װ�õ������ԣ�
����ȡ10.00 mL 1 mol��L-1������ʹ�����Һ�ֱ��������װ�õ���ƿ�У�
�ܳ�ȡ����������Ϊa g��þ��(��ȥ��������Ĥ���������ͬ)�����̶������ϸ�ĩ�ˣ�
��ͬʱ�����ϸ������ƶ���ʹþ��������������Ӧ��ȫ��
��Ӧ��������¶Ȼָ������£�������Ͳ��ˮ�����ΪV mL��
�������������ش��������⣺
(1)�����ֱ���������м���װ�������Եķ�����_____________________��
(2)��ȡ10.00 mL1mol��L-1����ʹ�����Һ�ֱ�ע����ƿ��ʱ�����õ�������_________(�����и��������ĸ)��
A.50 mL��Ͳ B.10 mL��Ͳ
C.25 mL��ʽ�ζ��� D.25 mL��ʽ�ζ���
(3)������У������ϳ�ȡ��þ��������������_________ g��
(4)����������¼��������_______��ʵ������й۲쵽����Ҫ������______________��
(5)������У���ȡ��Ͳ��ˮ�����ʱ�����Ӷ������Լ���������µ�����Ħ�����Ӱ��Ϊ_________(�ƫ��ƫС������ȷ����)��
(6)Ϊ��֤ʵ��˳�����У�������Ӧѡ�õĹ����_________(�������ĸ)��
A.100 mL B.200 mL C.500 mL
(7)��ˮ�����Ļӷ�Ӱ����Բ��ƣ��ڸ��¶Ⱥ�ѹǿ�£�����Ħ�����Ϊ______ L��mol-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�Ƹ��и�����ѧ����ĩ�������ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ijʵ��С��Ϊ�о��������ȡ�Ͳ�������ʣ���������ʵ�顣
ʵ��I:�Ʊ�����
ʵ������������������ˮ��Һ�Ʊ������װ����ͼ��ʾ(���ȡ�����������̶�װ�þ�����ȥ����ʵ��������£�
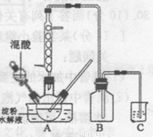
�ٽ�һ�����ĵ���ˮ��Һ��������ƿ��
�ڿ��Ʒ�ӦҺ�¶���55〜600C�����£��߽�������μ�һ�����������������Ļ��� (65%HNO3��98%H2S04��������Ϊ2 :1.5)��Һ
�۷�Ӧ3h���ң���ȴ�����˺����ؽᾧ�ò��ᾧ�塣
������������ˮ��Һ�����пɷ������з�Ӧ��
C6H12O6��12HNO3��3H2C2O4��9NO2����3NO����9H2O
C6H12O6��8HNO3��6CO2����8NO����10H2O
3 H2C2O4��2HNO3��6CO2����2NO����4H2O
(1)��������Ƿ�ˮ����ȫ�����õ��Լ�Ϊ________��
(2)ʵ����������μӹ��죬�����²�������½�����ԭ����________��
ʵ��II �����ᾧ���нᾧˮ�ⶨ
���ᾧ��Ļ�ѧʽ�ɱ�ʾΪH2C2O4 • xH2O,Ϊ�ⶨx��ֵ,��������ʵ�飺
�ٳ�ȡ6.3gij���ᾧ�����100.0mL��ˮ��Һ��
��ȡ25.00mL������Һ������ƿ�У���������ϡH2SO4����Ũ��Ϊ0. 5mol/L��KMnO4��Һ�ζ����ζ��յ�ʱ����KMnO4 �����Ϊ10.00mL���ش�����������
��3��д��������Ӧ�����ӷ���ʽ________________��
��4������x=________��
��5���ζ�ʱ�������ַ�Ӧ���ʿ�ʼ�����������ӿ죬���ܵ�ԭ����________��
ʵ��III:����ȶ���
�������ϣ����ᾧ��(H2C2O4 •xH20),1000C��ʼʧˮ��100.5�����ҷֽ����H2O��CO��CO2��������ͼ���ṩ���������Լ������һ��ʵ�飬֤�����ᾧ��ֽ�õ��Ļ��������H2O��CO��CO2 (����װ�ú͵��ܵ���ͼ����ȥ������װ�ÿ��ظ�ʹ�ã���

�ش��������⣺
(6)����װ�ð�����˳��Ϊ________��
(7)����B����ˮ����ͭ������________��
��8����֤��������к���CO��ʵ��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������У������һģ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��12�֣��������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֣�
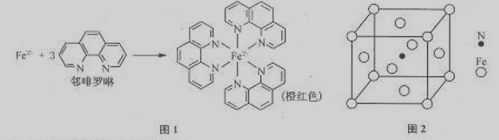
��1��Fe2+�ڻ�̬ʱ����������Ų�ʽΪ ��
��2���ǰ���NH2OH���в���sp3�ӻ���ԭ���� ���ǰ��۷е�ϸߣ�����Ϊ���Ӽ���ڽ�ǿ�� ��
��3��Fe2+���ڷ������γɵ������γɹ�����ͼ1���У���λ��Ϊ ��
��4�����ݼ۲㻥�����ۣ�C1O4���ռ乹��Ϊ ��
��5�������뵪�γ�һ�ִ��Բ��ϣ��侧���ṹ��ͼ2����ô��Բ��ϵĻ�ѧʽΪ ��
B����������Ǹ�֬���������������֬��״�ͨ��ȡ����Ӧ���������������������´����õ����ò������Ʊ�������͵IJ������£�
�ٽ�������ƿ����ƿ�����ﴦ��������������ƿ�м���20g�����ͣ��ٳ�ȡ40g�����飨Լ61mL����
�ڳ�ȡ�״�4.6g��Լ5.8mL���ŵ���ƿ�У�Ȼ���ȡ0.2g�������ƹ��岢ʹ֮�ܽ⣬Ȼ��ӵ�������ƿ�С�
������ͼ��ʾ��װ������ƿ��
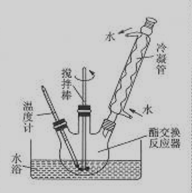
�ܺ���ˮԡ���ȣ�ʹ�¶ȱ�����60��65�����ң�����1.5��2h��
��ֹͣ���Ⱥ���ȴ��ȡ��������ƿ�����á���Һ���ϲ�
Ϊ�������ͣ�������ͼ״����²���ҪΪ���͡�
������ˮϴ���Ƶõ��������3��4�Ρ�
�߽�ˮϴ�����Һ����Բ����ƿ�У������¶ȱ�����
120�����ң�ֱ����Һ����������ƿ��ʣ���Һ����Ҫ��Ϊ
������͡�
��1���������Ƶ������� ��
��2��������������� ��
��3��ͼ�������ܵ������� ��
��4������ݷ�Һ���õ�����Ҫ��һ�ֲ��������� ��д���ƣ�
��5��ȷ���������ϴ�Ӹɾ��ķ����� ��
��6�����(1g����������֬���������������صĺ��������IJⶨ
a. ��ȡ��������Wgע����ƿ�У�����ʯ���ѡ��Ҵ����Һ25mL��ҡ����ƿʹ�����ܽ⡣
b.����3�η�̪����0.100 mol/L KOH��Һ�ζ���������ɫ�ұ���30s����ʧ������KOH��ҺVmL��
���������͵����Ϊ ���ú�W��V�Ĵ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com