
��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��109g5.51����NaOH��Һ��������CuSO4��Һ��200g10.00����K2SO4��Һ���缫��Ϊʯī�缫��
��ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47��������c�缫�������ӡ��ݴ˻ش����⣺
�� ��1���缫b�Ϸ����ĵ缫��ӦΪ___________________________________��
�� ��2���缫b�����ɵ������ڱ�״���µ����Ϊ__________________����ʱ���ձ���NaOH��Һ�����ʵ���Ũ��Ϊ������Һ���ܶ�Ϊ1g/cm3��_______________��
�� ��3���缫c�������仯��___________g����ʹ�������еĵ��Һ�ָ�����ʼ״̬��Ӧ������Һ�м���������___________������ĸ��ţ���
�� A. Cu(OH)2 ���� B.Cu2O�� �� C. CuCO3�� ��D. Cu2(OH)2CO3
�� ��4�������������䣬�������װ�ø�Ϊ��⾫��ͭ����c�缫�IJ���Ϊ___________��d�缫�IJ���Ϊ _____________��
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�������Ƕ��и߶���ѧ������������⻯ѧ�Ծ� ���ͣ������
��14�֣���ͼ��ʾװ���У��ס��������ձ��ֱ�����ʢ��200mL����ʳ��ˮ��������AgNO3��Һ��a��b��c��d�ĸ��缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ��������d�缫����������2.16g���ݴ˻ش����⣺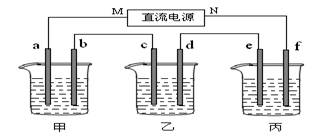
��1����Դ��N��Ϊ ����
��2���缫b�Ϸ����ĵ缫��ӦΪ ��
��3���缫c�����ɵ������ڱ�״̬�µ������ ��
��4������Һ������������Ũ��Ϊ ������Һ�����Ϊ200mL)��
��5�����ڱ��ձ���ʵ�����ı������һ��ͭ����������ҺΪ ��e�缫�IJ����ǣ� ��f�缫�ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����������ݶ��и����^��ѧ���Ի�ѧ���������� ���ͣ������
��12�֣�A��B��C����ǿ����ʣ�������ˮ�е�������������±���ʾ��
| ������ | Na+��K+��Cu2+ |
| ������ | SO42����OH�� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ��������ѧ�߶���ѧ�ڵ������¿���ѧ�Ծ����������� ���ͣ������
��10�֣���ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��100 g 5.00%��NaOH��Һ��������CuSO4��Һ��100 g 10.00%��K2SO4��Һ���缫��Ϊʯī�缫��


 ��1����ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӡ�
��1����ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӡ�
�ݴ˻ش����⣺ �ٵ�Դ��N��Ϊ ����
�ٵ�Դ��N��Ϊ ���� �ڵ缫b�Ϸ����ĵ缫��ӦΪ ��
�ڵ缫b�Ϸ����ĵ缫��ӦΪ �� �۵缫b�����ɵ������ڱ�״���µ������
�۵缫b�����ɵ������ڱ�״���µ������
 �ܵ缫c�������仯�� g��
�ܵ缫c�������仯�� g�� ��2�������������ͭȫ����������ʱ����ܷ�������У�Ϊʲô��
��2�������������ͭȫ����������ʱ����ܷ�������У�Ϊʲô��  ��
�� �ݵ��ǰ�����Һ��pH��α仯����������С�䣩
�ݵ��ǰ�����Һ��pH��α仯����������С�䣩 ����Һ ��
����Һ �� ����Һ�ߣߣߣߣߣߣߣ�
����Һ�ߣߣߣߣߣߣߣ� ����Һ�ߣߣߣߣߣߣߣ�
����Һ�ߣߣߣߣߣߣߣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�����и߶���ѧ������������⻯ѧ�Ծ� ���ͣ������
��14�֣���ͼ��ʾװ���У��ס��������ձ��ֱ�����ʢ��200mL����ʳ��ˮ��������AgNO3��Һ��a��b��c��d�ĸ��缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ��������d�缫����������2.16g���ݴ˻ش����⣺

��1����Դ��N��Ϊ ����
��2���缫b�Ϸ����ĵ缫��ӦΪ ��
��3���缫c�����ɵ������ڱ�״̬�µ������ ��
��4������Һ������������Ũ��Ϊ ������Һ�����Ϊ200mL)��
��5�����ڱ��ձ���ʵ�����ı������һ��ͭ����������ҺΪ ��e�缫�IJ����ǣ� ��f�缫�ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com