
����Ŀ��A��B��C��D��E����Ԫ��λ��Ԫ�����ڱ���ǰ�����ڣ�ԭ��������������AԪ�صļ۵����Ų�Ϊnsnnpn��1��BԪ��ԭ�������������Ǵ�����������3����Cλ��B����һ���ڣ��DZ���������õĽ���Ԫ�أ�D��̬ԭ�ӵ�3dԭ�ӹ���ϵĵ�������4sԭ�ӹ���ϵ�4����EԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӡ��ش���������(��Ԫ�ط��ű�ʾ��Ҫ������)��
(1)A��B��C�ĵ�һ��������С�����˳��Ϊ____________�����ߵ縺���ɴ�С��˳��Ϊ_________��
(2)A��E�ļ���̬�⻯��е�ߵ���______����ԭ����_________��
(3)D3����̬��������Ų�ʽΪ_________________��
(4)E��̬ԭ�ӵļ۵��ӹ����ʾʽΪ___________��
(5)B��E�γɷ��ӵĽṹ��ͼ��ʾ���÷��ӵĻ�ѧʽΪ_______��Eԭ�ӵ��ӻ�����Ϊ________��
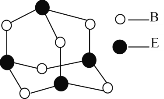
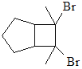
(6)B��C���γ����ӻ�����R���侧���ṹ��ͼ��ʾ��
��һ�������к�______��B���ӡ�R�Ļ�ѧʽΪ__________��
�ھ�������Ϊa pm������R���ܶ�Ϊ_____________gcm��3(ֻ�м���ʽ)��
���𰸡�Na��O��N O��N��Na NH3 �������Ӽ�����������Էе�� [Ar]3d7��1s22s22p63s23p63d7 ![]() As4O6 sp3 4 Na2O
As4O6 sp3 4 Na2O ![]()
��������
AԪ�صļ۵����Ų�Ϊnsnnpn��1����֪n=2��A�۵����Ų�ʽΪ2s22p3����ôA�ĺ�������Ų�ʽΪ1s22s22p3����AΪN��
BԪ��ԭ�������������Ǵ�����������3������BΪO��
Cλ��B����һ���ڣ��DZ���������õĽ���Ԫ�أ���CΪNa��
D��̬ԭ�ӵ�3dԭ�ӹ���ϵĵ�������4sԭ�ӹ���ϵ�4������D��̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2����ôD�����������=18+10=28��DΪNi��
EԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ���E��̬ԭ�ӵļ۵����Ų�ʽΪ��3d104s24p3����ôEԭ�Ӻ�����18+10+2+3=33�����ӣ�EΪ��As��
����������AΪN��BΪO��CΪNa��DΪNi��EΪAs���ݴ˷����ش�
(1) ��һ�������ǻ�̬����̬ԭ��ʧȥ������һ������������������һ��������ֵԽС��ԭ��Խ����ʧȥһ�����ӣ���һ��������ֵԽ��ԭ��Խ��ʧȥһ�����ӡ�һ����˵���ǽ�����Խǿ����һ������Խ������Na�ĵ�һ��������С��N�Ļ�̬ԭ�Ӵ��ڰ����״̬����ͬ�������ڵ�O�����ͣ����ȶ�������ʧ���ӣ�����N�ĵ�һ�����ܱ�O�����ߵĵ�һ�����ܹ�ϵΪ��Na��O��N���ǽ�����Խǿ���縺��Խ�����Ե縺�Թ�ϵΪ��O��N��Na���ʴ�Ϊ��Na��O��N ��O��N��Na��
(2)N��Asλ��ͬ���壬����̬�⻯��ΪNH3��AsH3��NH3����֮����������۷е��AsH3�ߣ��ʴ�Ϊ��NH3���������Ӽ�����������Էе�ߣ�
(3)Ni��28��Ԫ�أ�Ni3+������25�����ӣ����������Ų�ʽΪ��[Ar]3d7��1s22s22p63s23p63d7���ʴ�Ϊ��[Ar]3d7��1s22s22p63s23p63d7��
(4)EΪAs��AsΪ33��Ԫ�أ���̬ԭ�Ӻ�����33�����ӣ����̬ԭ�ӵļ۵��ӹ����ʾʽ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5)��ͼ��֪��1���÷��Ӻ�6��Oԭ�ӣ�4��Asԭ�ӣ��ʻ�ѧʽΪ��As4O6������Asԭ��![]() �����Ӷ���=
�����Ӷ���=![]() =3���¶Ե�����=
=3���¶Ե�����=![]() ���۲���Ӷ���=3+1=4������As4O6Ϊsp3�ӻ����ʴ�Ϊ��As4O6��sp3��
���۲���Ӷ���=3+1=4������As4O6Ϊsp3�ӻ����ʴ�Ϊ��As4O6��sp3��
(6)O��Na�ļ����ӣ�O�����Ӱ뾶��������ɫС�����O2-����ɫС�����Na+��
���ɾ�̯���ɵã�ÿ�������У�O2-����=![]() =4��Na+����=8�����ԣ�һ����������4��O2-��R�Ļ�ѧʽΪNa2O���ʴ�Ϊ��4��Na2O��
=4��Na+����=8�����ԣ�һ����������4��O2-��R�Ļ�ѧʽΪNa2O���ʴ�Ϊ��4��Na2O��
��![]() ��1�����������=(a pm)3=
��1�����������=(a pm)3=![]() �������ܶ�
�������ܶ� =
=![]() gcm��3���ʴ�Ϊ��
gcm��3���ʴ�Ϊ��![]() ��
��


 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾNaCl������ֱ�߽��㴦��ԲȦΪNa+��Cl-������λ�á������������ڿռ��������ഹֱ�ķ����϶��ǵȾ������еġ�

��1���뽫���д���Cl-��ԲȦͿ�ڣ����ؿ������Ӱ뾶��С���������NaCl����ʾ��ͼ��_____________
��2�������У���ÿ��Na+����Χ��������Ҿ�����ȵ�Na+����_______����
��3��һ��NaCl������Cl-�ĸ�������_______����______________�������ʽ����Na+�ĸ�������_______����______________�������ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ�ǰ�����ڵ�һ���֣�X��Y��Z��R��W��J��6��Ԫ�صĴ��š�����JΪ0��Ԫ�ء�
X | Y | Z | |
R | |||
W | |||
J |
����˵����ȷ���ǣ� ��
A.��̬Rԭ�ӵĹ����ʾʽΪ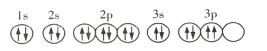
B.![]() ��
��![]() �İ뾶��С��ϵΪ
�İ뾶��С��ϵΪ![]()
C.Y�ĵ�һ�����ܴ���X�ĵ�һ������
D.X��Y��Z��R��W�У��縺������Ԫ��ΪW
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ�����Ϊ
��s��s ������s��p �����ĵ�������״��ͬ
�ڵ������ڵ�Ԫ�ػ�̬ԭ���У�4s�ܼ�ֻ��1�����ӵ�Ԫ�ع���3��
���ٵ����������[W(CO)5OH]���ܴ��̶�CO2����������������0��
������ԭ�Ӳ�ȡsp3�ӻ��ķ��ӣ������幹��һ������������
��2�������д�������̼ԭ��
����Է���������CH3CH2OH��CH3CHO�����Էе㣺CH3CH2OH��CH3CHO
A.�ڢۢ�B.�ۢ�C.�٢ڢ�D.�ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
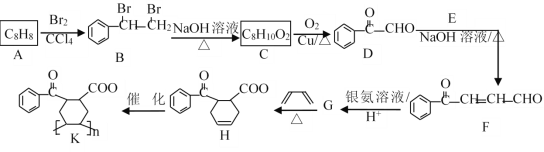
����Ŀ���л���KΪ�ϳɸ߷��ӻ����һ�ֺϳ�K�ĺϳ�·����ͼ��ʾ(���ֲ��P��Ӧ��������ȥ)��
��֪���� R1CHO+ R2CH2CHO![]()
![]() +H2O
+H2O
��![]() +
+![]()
![]()
![]()
�ش��������⣺
(1)A������Ϊ_________��F�к��������ŵ�����Ϊ___________��
(2)E��G�Ľṹ��ʽ�ֱ�Ϊ________��________��H��K�ķ�Ӧ����Ϊ_______��Ӧ��
(3)��C����D�Ļ�ѧ����ʽΪ___________��
(4)���������ϳ�·�ߺ���Ϣ���Լ�ȩ����ȩΪԭ��(���Լ���ѡ)�������ȡ����(������)�ĺϳ�·��Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ʊ�ͭ����һ�����ͽ��ܸ��³����壬�侧���ṹ��ͼ��ʾ���о����֣��˸��³������е�CuԪ�������ּ�̬���ֱ�Ϊ+2��+3��YԪ�صĻ��ϼ�Ϊ+3��BaԪ�صĻ��ϼ�Ϊ+2��

(1)�����ʵĻ�ѧʽΪ________��
(2)��������Cu2+��Cu3+�ĸ�����Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2L �����ܱ������г���X(g)��Y(g)��������ӦX(g)+Y(g)![]() M(g)+N(s)������ʵ���������±���
M(g)+N(s)������ʵ���������±���
ʵ�� ��� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | ||
n(X) | n(Y) | n(M) | n(N) | ||
�� | 800 | 0.10 | 0.40 | 0.080 | 0.080 |
�� | 800 | 0.20 | 0.80 | a | a |
�� | 900 | 0.10 | 0.15 | 0.06 | 0.06 |
����˵����ȷ����( )
A.ʵ��� 5min��ƽ�⣬ƽ����Ӧ����v(X)=0.016mol/(L��min)
B.ʵ����У��÷�Ӧ��ƽ�ⳣ��K=1
C.ʵ����У��ﵽƽ��ʱ��aС��0.16
D.����ӦΪ���ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��ij��ѧ������ͨ��![]() ������������Ʋ���мȺ�����λ�����ֺ����������ṹ������ͼ��ʾ��������λ��������������߱�ʾ��
������������Ʋ���мȺ�����λ�����ֺ����������ṹ������ͼ��ʾ��������λ��������������߱�ʾ��

��д����̬Cuԭ�ӵĺ�������Ų�ʽ��__��
��д��ͼ��ˮ��ͭ���ӵĽṹ��ʽ�����뽫��λ����ʾ��������__��
��2���ܶ�����л�����Ni�Ĵ������¿�����H2�����ӳɷ�Ӧ�����CH2=CH2����CH![]() CH����
CH����![]() ����HCHO�ȣ�����̼ԭ�Ӳ�ȡsp2�ӻ��ķ�����__������ţ����Ʋ�HCHO���ӵ����幹��Ϊ__�Ρ�
����HCHO�ȣ�����̼ԭ�Ӳ�ȡsp2�ӻ��ķ�����__������ţ����Ʋ�HCHO���ӵ����幹��Ϊ__�Ρ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������H��һ���л��������м��塣ʵ�����ɷ��㻯����A�Ʊ�H��һ�ֺϳ�·�����£�

��֪����![]()
��![]()
�ش��������⣺
(1)A�Ļ�ѧ������______________________��
(2)��C����D��E����F�ķ�Ӧ���ͷֱ���_____________________��____________________��
(3)E�Ľṹ��ʽΪ______________________________��
(4)GΪ�ױ���ͬ���칹�壬��F����H�Ļ�ѧ����ʽΪ______________________________��
(5)���㻯����X��F��ͬ���칹�壬X���뱥��̼��������Һ��Ӧ�ų�CO2����˴Ź���������ʾ��4�ֲ�ͬ��ѧ�������⣬�������Ϊ6:2:1:1��д��2�ַ���Ҫ���X�Ľṹ��ʽ��_______________��
(6)д���û������2-��ȲΪԭ���Ʊ�������ĺϳ�·��(�����Լ���ѡ) _______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com