
ij����Һ�п��ܺ���Ag+��Fe3+��K+��Ba2+��NH4+�����ӣ���������ʵ�飺
����ô���Һ�м��������ϡ���ᣬ�а�ɫ�������ɡ�
�ڹ��ˣ�����Һ�м��������ϡ���ᣬ���а�ɫ�������ɡ�
�۹��ˣ�ȡ������Һ������2��KSCN��Һ��û�����Ե�������֡�
����ȡ����������е���Һ������NaOH��Һ����Һ�ʼ��ԣ����ȣ��ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����塣����ʵ������ó��Ľ����У���ȷ���ǣ� ��
A. ����Һ��һ������Ba2+��Fe3+ B. ����Һ��һ������Ag+��Fe3+��NH4+
C. ����Һ��һ��������Ba2+ D. ����Һ����ȷ�������ӿ�����ɫ��Ӧ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ���Ĵ�ʡ�㰲���������ڽ���üɽ����2017������ڶ�����Ͽ������ۻ�ѧ�Ծ� ���ͣ������
����п������������Ͻ����ִ�����е���;Խ��Խ�㷺��
(1)����Ԫ�����ڱ��е�λ����_____________����̬��ԭ����_________�ֲ�ͬ�˶�״̬�ĵ��ӡ�
(2)������CO�γ������Ni(CO)n��CO�����ЦҼ���м��ĸ�����Ϊ________�����������ԭ��Ni�ļ۲��������Ϊ18����n=________�����������۵���170�棬��Ni(CO)n ����__________
(3)�ڸ��Ĵݻ������£��Ҵ��ɱ���������Ϊ��ȩ(CH3CHO������ȩ������̼ԭ�ӵ��ӻ���ʽ��______����ȩ������H��C=O�ļ���_______������ڡ��������ڡ���С�ڡ����Ҵ������е�H��C��O�ļ��ǡ��Ҵ�����ȩ����������ˮ������Ҫԭ����_______________��
(4)����NiO����ṹ������NaCl����Ni2+����O2-���ɵ�________��϶����������塱�����������塱���������塱��ѹ������塱�����侧���߳�Ϊ�� pm����ʽ��ʾNiO������ܶ�Ϊ____g/cm3�����ؼ��������������ӵ�������ֵΪNA��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ��ͨ�и����߿�ȫ��ģ�⣨�壩��ѧ �Ծ��������棩 ���ͣ�ѡ����
һ���¶��������Ϊ2L�ĺ����ܱ������г���6 molCO2��8mo1H2��������Ӧ��CO2 (g)+3 H2(g)= CH3OH(g)+H2O(g) ��H=-49kJ��mol-1�����n(H2)���ʵ�����ʱ��仯������I ��ʾ������˵����ȷ���ǣ� ��

A. �÷�Ӧ��O��8min�ڵ�v(CO2)=0.125mol��L-1��min-1
B. ����ʼʱ�������г���3mo1CO2��4molH2����ƽ��ʱCH3OH�������������20%
C. ����ʼʱ�������г���4mo1CO2��2molH2��2molCH3OH��1molH2O(g)�����ʱ��Ӧv(��)��v(��)
D. ����II������III�ı������ֱ��������¶ȡ���Сѹǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������к�����2017�����һģ��ѧ�Ծ� ���ͣ������
�Է�����AΪԭ�Ϻϳ��л���F��I�ĺϳ�·�����£�

��1��A�ķ���ʽΪ__________��C�еĹ���������Ϊ________________��
��2��D�����������______��ԭ�ӹ�ƽ�档
��3��E����F�ķ�Ӧ����Ϊ___________��G�Ľṹ��ʽΪ__________��
��4����H����I�Ļ�ѧ����ʽΪ______________��
��5����������������B��ͬ���칹����_______�֣������������칹�������к˴Ź�������Ϊ4��壬�������Ϊ6��2��1��1����________��д������һ�ֵĽṹ��ʽ����
�����ڷ��㻯��� ���ܷ���������Ӧ��
��6����֪ �����������ϳ�·�ߣ��Ա��ͱ���Ϊԭ�ϣ����Լ���ѡ��������Ʊ�
�����������ϳ�·�ߣ��Ա��ͱ���Ϊԭ�ϣ����Լ���ѡ��������Ʊ� �ĺϳ�·��________________��
�ĺϳ�·��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������к�����2017�����һģ��ѧ�Ծ� ���ͣ�ѡ����
����˵����ȷ����
A. FeSO4��Һ����ڼ����������۵��Լ�ƿ��
B. ��1mol/L��NaCl��Һ����������ƿ��
C. ���Ǹ������������ȣ���Ҫ��ʯ�������Է�����ը��
D. ��pH��ֽ����ij��Һ�������ʱ��һ��Ҫ��������ˮʪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ���ݰ�һ�С�Ȫ��ʵ����ѧ��һ��ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ѡ����
���������м��ܸ�ϡ���ᷴӦ�����ܸ�����������Һ��Ӧ���ǣ� ��
��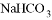 ��
��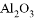 ��
�� ��
��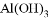 ��MgO ��Fe(OH) 3 ��CuSO4
��MgO ��Fe(OH) 3 ��CuSO4
A. ȫ�� B. �ڢۢܢ�
C. �٢ڢۢ� D. �٢ڢۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ���ݰ�һ�С�Ȫ��ʵ����ѧ��һ��ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ѡ����
����һ�����ʵ���Ũ�ȵ�NaOH��Һʱ�����������ҺŨ��ƫ�͵�ԭ����
A��δϴ���ձ��Ͳ����� B��ת����Һǰ��Һδ��ȴ������
C������ƿδ���� D������ʱ����Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ��һ��ѧ�ڵ�һ�Σ�3�£��μ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
ͼ1���ڽ���п��������һ����ij��Һ��ʪ����ֽ��ͼ2��NaBH4/H2O2ȼ�ϵ�أ�������˵����ȷ����

A��ͼ2��طŵ�����У�Na��������������Ǩ��
B��ͼ2��ظ������ĵ缫��ӦΪBH4���D 8e�� + 8OH����BO2�� + 6H2O
C�����������ƺͷ�̪�Ļ����Һ��ʪ��ֽ���õ��߽�a��bֱ����������Ǧ��оC�㴦���ֺ�ɫ
D������KI������Һ��ʪ��ֽ���õ��߽�a��b��A��B�缫������Ǧ��оC�㴦������ɫ����b�ӵ���A�缫
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶���ѧ�ʼ죨3�£���ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪H2��g����C2H4��g����C2H5OH��l����ȼ���ȷֱ���285.8 kJ��mol��1��1 411.0 kJ��mol��1��1 366.8 kJ��mol��1������C2H4��g����H2O��l����Ӧ����C2H5OH��l���Ħ�HΪ�� ��
A����44.2 kJ��mol��1 B����44.2 kJ��mol��1 C����330 kJ��mol��1 D����330 kJ��mol��1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com