
(1) ��A��B�����������ǵ������ͬ����Լ��85.7%��̼����A������������ܶ���28����Bʽ���ȿ�����ƽ��ʽ����С�������ʽ��A��ͬ����A��B����ʹ������Ȼ�̼��Һ��ɫ����������ʵ����ʵ���㲢�ش����⡣
������A��B�����Ļ�ѧʽ�������㲽��д�����棩
A ��B ��
��A��B�� ����A��B�Ľṹ��ʽ������ͬ���칹�塣
��д��B��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽ��
��
��2����֪ �ɼ�дΪ
�ɼ�дΪ ��
��
����ij������W�ķ��ӽṹ�ɱ�ʾΪ��![]() ��
��
��W�ķ���ʽΪ ��
��W��һ�ȴ����� �֡�
�������й�W��˵������ȷ���� �����ţ���
a.�ܷ�����ԭ��Ӧ b���ܷ���������Ӧ c���ܷ����Ӿ۷�Ӧ
d����������W�뱽�ֱ���ȫȼ�������ĵ���������ǰ�ߴ�
��д��W�ķ�����ͬ���칹�壨�ܷ����ۺϷ�Ӧ���Ľṹ��ʽ ���þۺϷ�Ӧ�Ļ�ѧ����ʽΪ ��
��W���� �����ţ���
a�������� b������ c���������� d��Ȳ��
��15�֣�
(1)��7�֣� ��A��C2H4 ��2�֣� B.C4H8(2��)
��B C4H8��1�֣�
��CH2��CH2+ Br2 = CH2 Br CH2 Br��2�֣�
�Ƶ�����:��A��H2������ܶ�Ϊ28����ʽ��Ϊ56,���ݺ�̼��85.7%������仯ѧʽ�к�4��̼��ΪC4H8����B��ʽ���ȿ���С��ӦС��29,����A�����ʽ��ͬ��ΪC2H4��
��2����8�֣���C8H8 ��1�֣� ��2 ��1�֣� ��d��1�֣�
![]() ��
��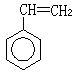 ��1�֣��� n
��1�֣��� n
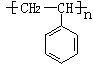
��2�֣�
��b��c ����1�֣���2�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com