
��1��������ǡ��ȫ���ܽ⣬������������ΪV����V��ȡֵ��ΧΪ_________________��
��2����������Һ��������Ӧ����Һ�����Ϊ1 L�������Һ��Fe 2+Ũ��Ϊ0.1 mol��L -1����ԭʼ������к�ͭ������Ϊ__________________________��
0.3 L��V��0.4 L��14.4 g��3.2 g
����������ȫ���ܽ⣬�漰�ķ�Ӧ�У�
��Fe2O3+3H2SO4�T�TFe2(SO 4)3+3H2O
��Fe2(SO 4)3+Cu�T�T2FeSO 4+CuSO 4
�������ֻ��Fe2O3����Ӧ�����ٽ��У���Ȼǡ�÷�Ӧʱ��Ӧ���㣺1 mol��L -1��V=3��22.4 g/160 g��mol -1�����V=0.42 L��
���������Cu��Fe2O3�Ļ�����Ӧ���٢���ʽ���У���Ҫ����ǡ���ܽ⣬���ʵ���Ӧ���㣺n(Cu)=n��Fe2(SO4)3��=n(Fe2O3)=x�������⣺64 g��mol -1x+160 g��mol -1x=22.4 g,���x=0.1 mol��
�ɷ�Ӧ�ٵù�ϵʽ1 mol��L -1��V=3��0.1 mol, V=0.3 L��?
����V��ȡֵ��Χ�ǣ�0.3 L��V��0.42 L
��2����Һ�У�n(Fe 2+)=0.1mol �������ɷ�Ӧʽ�����ɵģ���ΪFe2(SO 4)3��Cu˭��������Ŀ�в���ȷ������Ҫ�������ۡ�
��Cu��������n(Fe2O3)=n��Fe2(SO4)3��=1/2n(Fe2SO 4)=0.05 mol ��Cu��������22.4 g-m(Fe2O3)=22.4 g-0.05 mol��160 g��mol -1=14.4 g
��Fe2(SO 4)3��������n(Cu)=1/2n(FeSO 4)=0.05 mol ��Cu������Ϊ0.05 mol��64 g��mol -1=3.2 g
�������ۣ�ԭ�������ͭ������Ϊ14.4 g��3.2 g��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| ||
| ||
| 168Q |
| m |
| 168Q |
| m |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

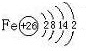
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�츣��ʡ�����и�����ͨ���б�ҵ��������飨���ۣ���ѧ���� ���ͣ������
������������������������������в����Ĺ�ҵ��������Ҫ��Fe2O3������SiO2��Al2O3��CaO��MgO�����ʣ����ø�������ȡҩ�ø��ϡ������������Ĺ����������£�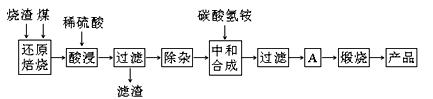
��1���ڡ���ԭ���ա��в������ж���������� ��
��2���������ʱ��һ�㲻����20 min�����ڿ��������ʱ���������Һ��Fe2+�������½�����ԭ�������ӷ���ʽ��ʾ�� ��
��3�������±����ݣ�
| �������� | Al(OH)3 | Mg(OH)2 | Fe(OH)3 | Fe(OH)2 |
| ��ʼ������pH | 3.10 | 8.54 | 2.01 | 7.11 |
| ��ȫ������pH | 4.77 | 11.04 | 3.68 | 9.61 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com