
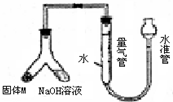
��12�֣�ij������ȤС��Ϊ̽��ij�����Ͻ𣨺�Mg Al�����������������ͼװ�ý���ʵ�顣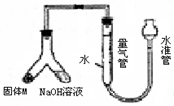
��1����μ����װ�õ�������
��2����Ʒ������������Һ��Ӧ�����ӷ���ʽ: ��
��3��ʹ��������������Һ��ag�Ͻ�(����M)��ַ�Ӧ�����������ܵ��������ΪVmL
(�ѻ���ɱ�״������ͬ)����a��0.036��V��22.4���úϽ����������������� ��
��4������װ���е����������滻Ϊ���������ᣬ��ag�Ͻ��ĩ��ַ�Ӧ��������������ڵ�������� (�����������������)VmL�����ô˷�����õ���������ܷ�������ĺ������㣿 ����ܡ���
��12�֣�
��1����װ��װ�ò����������ڼ�ˮ�����ϣ��£��ƶ�ˮ�ܣ�����Ƭ�̣���ˮ����Һ����������Һ��ά��һ���ĸ߶Ȳ˵��װ���ܷ�(����������)��3�֣�
��2��2OH��+2Al+6H2O�� 2 [AI(OH)4]��+ 3H2�� (2��)
����2OH��+2Al+2H2O �� 2 AlO2��+ 3H2����
��3��50%��0.5��3�֣� ��4���� (2��) �� (2��)
�����������������ʵ��Ŀ�ĺ�װ��ͼ֪�����������������Ʒ�Ӧ����������һԭ�����ⶨ��������
��1��Ҫ��������װ�õ������ԣ������һ��Ҫ��գ�һ����ˮ���ܷ⣬����ѹǿ�������飬��װ�ÿ��Բ������������ڼ�ˮ�����ϣ��£��ƶ�ˮ�ܣ�����Ƭ�̣���ˮ����Һ����������Һ��ά��һ���ĸ߶Ȳ˵��װ���ܷ⣻
��2����������������Ӧ����ƫ�����ƺ�������2OH��+2Al+2H2O �� 2 AlO2��+ 3H2����2OH��+2Al+6H2O�� 2 [AI(OH)4]��+ 3H2��
��3�����ݷ���ʽ����������������������ʵ�����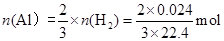 ������������
������������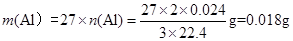 ��
��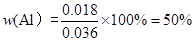
��4������������þ��������Ӧ�������������������������amL����Ϊ֪����������������֪�������������������������������������������þ��������
���㣺ͨ���ⶨ���������������������Եļ��鷽����������Ӧԭ����ѧ����ʵ�����ݵĴ���������
�����������ۺ��Խ�ǿ���Ѷ��еȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij������ȤС��Ϊ̽��ij�����Ͻ𣨺Ͻ�Ԫ��ΪMg Al���Ƿ���Ϲ��������������ҹ涨�������������ܵ���78%���������ͼװ�ý���ʵ�飮
ij������ȤС��Ϊ̽��ij�����Ͻ𣨺Ͻ�Ԫ��ΪMg Al���Ƿ���Ϲ��������������ҹ涨�������������ܵ���78%���������ͼװ�ý���ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij������ȤС��Ϊ̽��ij�����Ͻ𣨺�Mg Al�����������������ͼװ�ý���ʵ�飮
ij������ȤС��Ϊ̽��ij�����Ͻ𣨺�Mg Al�����������������ͼװ�ý���ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
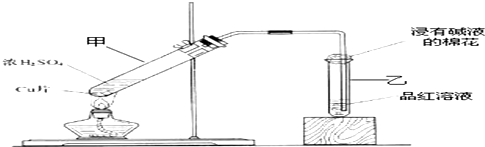 ij������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������������ͼ��ʾװ�ý����й�ʵ�飺
ij������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������������ͼ��ʾװ�ý����й�ʵ�飺
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��12�֣�ij������ȤС��Ϊ̽��ij�����Ͻ𣨺�Mg Al�����������������ͼװ�ý���ʵ�顣
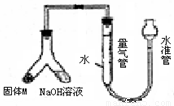
��1����μ����װ�õ�������
��2����Ʒ������������Һ��Ӧ�����ӷ���ʽ: ��
��3��ʹ��������������Һ��ag�Ͻ�(����M)��ַ�Ӧ�����������ܵ��������ΪVmL
(�ѻ���ɱ�״������ͬ)����a��0.036��V��22.4���úϽ����������������� ��
��4������װ���е����������滻Ϊ���������ᣬ��ag�Ͻ��ĩ��ַ�Ӧ��������������ڵ�������� (�����������������)VmL�����ô˷�����õ���������ܷ�������ĺ������㣿 ����ܡ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com