
��15�֣�������ʵ���������ÿ�����þ��Ϊԭ����ȡ��������þ(Mg3N2)����֪ʵ���п��ܻᷢ�����з�Ӧ����2Mg+O2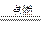 2MgO����3Mg+N2
2MgO����3Mg+N2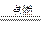 Mg3N2��
Mg3N2��
��2Mg+CO2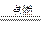 2MgO+C ��Mg+H2O
2MgO+C ��Mg+H2O MgO+H2�� ��Mg3N2 +6H2O ===3Mg(OH)2+2NH3��
MgO+H2�� ��Mg3N2 +6H2O ===3Mg(OH)2+2NH3��
�ɹ�ѡ���װ�ú�ҩƷ����ҳͼ��ʾ(þ�ۡ���ԭ���۾��Ѹ��װ�����������ķ�Ӧ����ȫ�ģ�����װ�õ�ĩ������������)��
�ش��������⣻
(1) �����ʵ�鷽��ʱ����װ��A��E�⣬��Ӧѡ���װ��(����ĸ����)����Ŀ�ķֱ�Ϊ���ɲ�������
| װ�� | Ŀ�� |
| | |
| | |
| | |
| | |
 ��
�� ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ�����������ѧ2009�컯ѧ�������¿����� ���ͣ�058
������ʵ���������ÿ�����þ��Ϊԭ����ȡ��������þ(Mg3N2)����֪ʵ���п��ܻᷢ�����з�Ӧ��
��2Mg��O2![]() 2MgO��
2MgO��
��3Mg��N2![]() Mg3N2��
Mg3N2��
��2Mg��CO2![]() 2MgO��C
2MgO��C
��Mg��H2O![]() MgO��H2��
MgO��H2��
��Mg3N2��6H2O![]() 3Mg(OH)2��2NH3��
3Mg(OH)2��2NH3��
�ɹ�ѡ���װ�ú�ҩƷ����ͼ��ʾ(þ�ۡ���ԭ���۾��Ѹ��װ�����������ķ�Ӧ����ȫ�ģ�����װ�õ�ĩ������������)��

�ش��������⣻
(1)�����ʵ�鷽��ʱ����װ��A��E�⣬��Ӧѡ���װ��(����ĸ����)����Ŀ
________��________________________________
________��________________________________
________��________________________________
________��________________________________
(˵�������Բ�����������������Ҳ�������ӣ�)
(2)ͨ�������ͬʱ��ȼA��Fװ�õľƾ��ƣ���ʵ�����к�Ӱ�죿________��ԭ����________��
(3)�����һ����Сʵ�飬������֤�����ǵ���þ��________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com