
��7�֣�(1)��֪��H��H����Ϊ436kJ/mol��N��N����Ϊ945kJ/mol��N��H����Ϊ391kJ/mol�����ݼ��ܼ��㹤ҵ�ϳɰ��ķ�Ӧ��___________ ��Ӧ(����ȡ����ȡ�)������1mol N2�ϳɰ���Ӧ�� =______________��
=______________��
(2) �ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
��Fe2O3(s)+3CO(g) ��2Fe(s)+3CO2(g) ��H���D24.8kJ��mol
��3Fe2O3(s)+ CO(g) ��2Fe3O4(s)+ CO2(g) ��H���D47.2kJ��mol
��Fe3O4(s)+CO(g) ��3FeO(s)+CO2(g) ��H��+640.5kJ��mol
��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽΪ ��
��1�� ����(2��)����93KJ/mol (2��)
��2��CO (g)��FeO(s)=" Fe(s)+" CO2 (g) ��H=��218KJ/mol (3��)
���������������1����Ӧ�ȵ��ڷ�Ӧ����ܼ��ܼ�ȥ��������ܼ���945kJ/mol+3��436KJ/mol-6��391kJ/mol=��93KJ/mol ��ͨ�����㷴Ӧ�ġ�H����0Ϊ���ȷ�Ӧ����2�������Ȼ�ѧ����ʽΪ-��-��-2���ۣ���CO (g)��FeO(s)=" Fe(s)+" CO2 (g)����H����Ӧ�ļ��㣬��H=��218KJ/mol��
���㣺�Ȼ�ѧ����ʽ����д����Ӧ�ȵļ���
��������������ע���˹���ɵ�Ӧ�á����ڼ��⡣


 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ǵ�ѭ���е���Ҫ���ʣ����ĺϳ���Ŀǰ�ձ�ʹ�õ��˹��̵�������
(1)��֪��H��H����Ϊ436 kJ��mol��1��N��N����Ϊ946 kJ��mol��1��N��H����Ϊ391 kJ��mol��1��д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ��
(2)�����£���һ��4L���ܱ������г���5.2molH2��2molN2����Ӧ�����ж�NH3��Ũ�Ƚ��м�⣬�õ����������±���ʾ��
| ʱ��/min | 5 | 10 | 15 | 20 | 25 | 30 |
| c(NH3)/mol��L��1 | 0.08 | 0.14 | 0.18 | 0.20 | 0.20 | 0.20 |
��5min�ڣ�����H2��ƽ����Ӧ����Ϊ___________________________________
��N2��ƽ��ת����
�۴������¸÷�Ӧ�Ļ�ѧƽ�ⳣ��K=__________________________����Ӧ�ﵽƽ�����ά������������䣬�¶Ȳ��䣬��ƽ����ϵ�м���H2��N2��NH3��8mol����ѧƽ�⽫��_______�����ƶ�(�����Ӧ�����淴Ӧ��)��
����ά������������䣬ֻ�����¶Ȼ�ѧƽ�ⳣ��ֵ ������С�����䣩������Ӧ���� ������С�����䣩��
�����¶�ά�ֲ��䣬ֻ�����������4L��Ϊ2L������ƽ��ʱN2��ƽ��Ũ�� 0.4 mol��L��1������ڡ�С�롢���ڣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�츣��ʡ����˫ʮ��ѧ����12���¿���ѧ�Ծ� ���ͣ������
���ǵ�ѭ���е���Ҫ���ʣ����ĺϳ���Ŀǰ�ձ�ʹ�õ��˹��̵�������
(1)��֪��H��H����Ϊ436 kJ��mol��1��N��N����Ϊ946 kJ��mol��1��N��H����Ϊ391 kJ��mol��1��д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ��
(2)�����£���һ��4L���ܱ������г���5.2molH2��2molN2����Ӧ�����ж�NH3��Ũ�Ƚ��м�⣬�õ����������±���ʾ��
| ʱ��/min | 5 | 10 | 15 | 20 | 25 | 30 |
| c(NH3)/mol��L��1 | 0.08 | 0.14 | 0.18 | 0.20 | 0.20 | 0.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ�߶��^����������ѧ�Ծ��������棩 ���ͣ������
��7�֣�(1)��֪��H��H����Ϊ436kJ/mol��N��N����Ϊ945kJ/mol��N��H����Ϊ391kJ/mol�����ݼ��ܼ��㹤ҵ�ϳɰ��ķ�Ӧ��___________ ��Ӧ(����ȡ����ȡ�)������1mol N2�ϳɰ���Ӧ��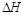 =______________��
=______________��
(2) �ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
��Fe2O3(s)+3CO(g) ��2Fe(s)+3CO2(g) ��H���D24.8kJ��mol
��3Fe2O3(s)+ CO(g) ��2Fe3O4(s)+ CO2(g) ��H���D47.2kJ��mol
��Fe3O4(s)+CO(g) ��3FeO(s)+CO2(g) ��H��+640.5kJ��mol
��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�긣��ʡ����12���¿���ѧ�Ծ� ���ͣ������
���ǵ�ѭ���е���Ҫ���ʣ����ĺϳ���Ŀǰ�ձ�ʹ�õ��˹��̵�������
(1)��֪��H��H����Ϊ436 kJ��mol��1��N��N����Ϊ946 kJ��mol��1��N��H����Ϊ391 kJ��mol��1��д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ��
(2)�����£���һ��4L���ܱ������г���5.2molH2��2molN2����Ӧ�����ж�NH3��Ũ�Ƚ��м�⣬�õ����������±���ʾ��
|
ʱ��/min |
5 |
10 |
15 |
20 |
25 |
30 |
|
c(NH3)/mol��L��1 |
0.08 |
0.14 |
0.18 |
0.20 |
0.20 |
0.20 |
��5min�ڣ�����H2��ƽ����Ӧ����Ϊ___________________________________
��N2��ƽ��ת����
�۴������¸÷�Ӧ�Ļ�ѧƽ�ⳣ��K=__________________________����Ӧ�ﵽƽ�����ά������������䣬�¶Ȳ��䣬��ƽ����ϵ�м���H2��N2��NH3��8mol����ѧƽ�⽫��_______�����ƶ�(�����Ӧ�����淴Ӧ��)��
����ά������������䣬ֻ�����¶Ȼ�ѧƽ�ⳣ��ֵ ������С�����䣩������Ӧ���� ������С�����䣩��
�����¶�ά�ֲ��䣬ֻ�����������4L��Ϊ2L������ƽ��ʱN2��ƽ��Ũ�� 0.4 mol��L��1������ڡ�С�롢���ڣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com