
��8�֣���1��ʵ�����������Ȼ�����Һʱ���������������ǣ�ԭ�����ӷ���ʽ��ʾΪ ������������������Ϊ��ֹ��������������������ʱ������
(2)��������2���Ȼ�ѧ��Ӧ����ʽ��
FeO(s)+CO(g)=" Fe(s)+" CO2(g) ��H= �D218kJ��mol
Fe3O4(s)+CO(g)==3FeO(s)+CO2(g) ��H= +640.5kJ��mol
д��CO���廹ԭFe3O4����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��
______________
��4�������£������ʵ�����Ũ�ȵĢٰ�ˮ ��NH4HSO4 ��NH4Cl ��(NH4)2CO3
��(NH4)2SO4��Һ�У�c(NH4+)�ɴ�С��˳��Ϊ_____________________________(�����)
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
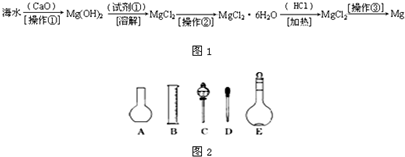
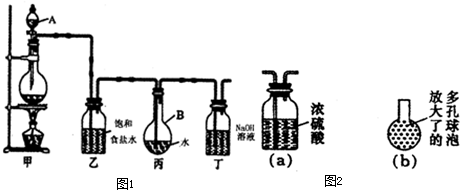
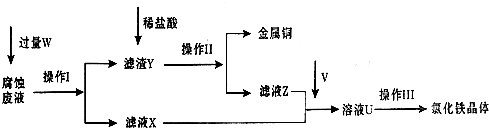
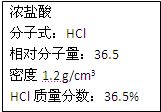 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ������и����ڶ����¿���ѧ�Ծ� ���ͣ�ʵ����
��15�֣�
������������Ҫ������������ԭ��������ˮ��Һ�ֳ�Ϊ˫��ˮ��ij��ѧ��ȤС��ȷ�ⶨ�˹�������ĺ�������̽���˹�����������ʡ�
��.�ⶨ��������ĺ���
��1�� ʵ����ȷ����250mL����������Һ, ����Ҫ�õ�������ƽ���ձ�����Ͳ��ҩ�ס��������⣬�������õ���������___________�� ��(����������)��ȡ���ƺõĹ���������Һ25.00mL����ƿ�У�����ϡ�����ữ��������������
��2�� �ø�����ر���Һ�ζ������������䷴Ӧ�����ӷ���ʽ���£��뽫������ʵĻ�ѧ����������ѧʽ��д�ڷ����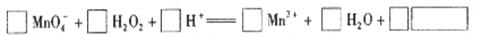
(3) �ζ�ʱ����������ر���Һע��___________________�����ʽ����ʽ�����ζ����С��ζ������յ��������_________________ ______��
��4�� �ظ��ζ����Σ�ƽ������c mol/L KMnO4����ҺV mL����ԭ����������Һ�й����������������Ϊ_________________________________��
��5�� ���ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ����ⶨ���_________���ƫ�ߡ���ƫ�͡����䡱����
��.̽���������������
�����������ṩ��ʵ�������������ʵ��:�ֱ�֤����������� �����ԺͲ��ȶ��ԡ���ʵ���������Լ�ֻ�й���������Һ����ˮ���⻯�ص�����Һ������������Һ��ʵ����������Ʒ����ѡ����
�����ԺͲ��ȶ��ԡ���ʵ���������Լ�ֻ�й���������Һ����ˮ���⻯�ص�����Һ������������Һ��ʵ����������Ʒ����ѡ����
�뽫���ǵ�ʵ�鷽����ʵ�����������±���
| ʵ������ | ʵ�鷽�� | ʵ������ |
| ��֤������ | | |
| ��֤���ȶ��� | | |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com