
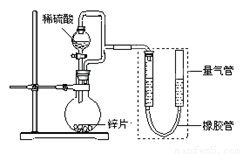
(15��) ijͬѧ���������ͼ��ʾװ�ã����ּг�װ������ȥ������װ�ÿ����������ж���
ʵ���о���
��ش�
��1��������װ��̽��Ӱ�컯ѧ��Ӧ���ʵ����ء�
��Բ����ƿ�з�����Ӧ�����ӷ���ʽ�� ��
��������װ�ý���ʵ�飬������9.0 mL����Ϊ��ʱ�յ㣬���Ϊt1��t2��
|
��� |
V(H2SO4)/mL |
c(H2SO4)/mol��L��1 |
t/s |
|
�� |
40 |
1 |
t1 |
|
�� |
40 |
4 |
t2 |
�Ƚ�ʵ���͢���Եó���ʵ������� ��
������пƬ���ɺ����ʵĴ�пƬ���ҿ�����������ʹ��������ʵ����ȫһ�£�����õķ�Ӧ���ʾ���������ʵ���Ӧ�����ݡ���пƬ���������ʿ����ǣ�����ţ� ��
a��ʯī b���� c��ͭ d��ɳ�����������裩
��2��������װ�òⶨ��пƬ�Ĵ��ȡ�
�����Ӻ�װ�ã����װ�������ԣ�������пƬmg����Բ����ƿ�У��μ�����ϡ���ᣬ��ַ�Ӧֱ�����ٲ�������Ϊֹ������������ΪVL�����пƬ�Ĵ���Ϊ ��
�ڱ�ʵ���ڶ���ǰ�������ܵIJ����� ��
�۱���װ���������ǵ����ϡ��������������������������ȷ�������õ����������� �����ƫ����ƫС������Ӱ�족����
��3��������װ����֤�����ڳ�ʪ�����лᷢ��������ʴ��
��Բ����ƿ�е��Լ���ѡ�ã�����ţ� ��
a��NH4Cl��Һ b��C2H5OH c��Na2CO3��Һ d��ϡ����
����֤�������ڳ�ʪ�����лᷢ��������ʴ�������� ��
��15�֣���1����Zn+2H+ ��Zn2++H2����1�֣�
������������һ��ʱ����ѧ��Ӧ�����淴Ӧ��Ũ�ȵ����������2�֣���abc��2�֣�
��2����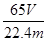 ��100%��2�֣�
��100%��2�֣�
�ڴ�������ȴ�����ұ������ܸ߶ȣ�ʹ�����ҹ���ˮ����ƽ��2�֣� ����Ӱ�죨2�֣�
��3����c ��2�֣� ����������ܵ�ˮ���������ҹܵ�ˮ���½���2�֣�
��������
�����������1����п��ϡ���ᷴӦ�����ӷ���ʽΪZn+2H+ ��Zn2++H2����
�ڸ��ݱ������ݿ�֪��ʵ���͢���ȣ�ʵ����������Ũ�ȴ���ʱ�٣���˵������������һ��ʱ����ѧ��Ӧ�����淴Ӧ��Ũ�ȵ����������
������õķ�Ӧ���ʾ���������ʵ���Ӧ�����ݣ���˵����пƬ������������п������ԭ��أ������ʵĽ���������п�ģ����Է�����������ʯī������ͭ����ѡabc��
��2��������������ڱ�״������VL�������������ʵ�����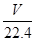 mol������ݷ���ʽ��֪���μӷ�Ӧ��п�����ʵ�����
mol������ݷ���ʽ��֪���μӷ�Ӧ��п�����ʵ�����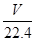 mol����������
mol����������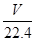 mol��65g/mol��
mol��65g/mol��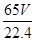 g�����Դ�пƬ�Ĵ���Ϊ
g�����Դ�пƬ�Ĵ���Ϊ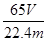 ��100%��
��100%��
�����������������¶Ⱥ�ѹǿӰ��������ڶ���ǰ�������ܵIJ����Ǵ�������ȴ�����ұ������ܸ߶ȣ�ʹ�����ҹ���ˮ����ƽ��
������װ���Ƿ�յģ�����ϡ��������������Ӱ����������ܵ������������˶�ʵ������Ӱ�졣
��3����Ҫ������װ����֤�����ڳ�ʪ�����лᷢ��������ʴ����������Һ������Ӧ�ú��������������Ի���ԡ��Ȼ������ˮ�����ԣ�ϡ��������ˮ�����ԣ��Ҵ��Ƿǵ���ʣ�̼������Һ�Լ��ԣ����Դ�ѡc��
���������������ʴ����װ����ѹǿ���ͣ�������֤�������ڳ�ʪ�����лᷢ��������ʴ����������������ܵ�ˮ���������ҹܵ�ˮ���½���
���㣺������������Է�Ӧ���ʵ�Ӱ�졢���Ȳⶨ����������ⶨ��������������ʴ�Լ�ʵ�鷽����������۵�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�ij�һ�и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��4�֣�I. ����ʵ��������ʵ����ʵ����������ȷ���� ������ţ�
�ٷ�Һ����ʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ���
������ڵ�NaOHϡ��Һ�еμ�FeCl3������Һ�����Ʊ�Fe(OH)3����
��ʯ�͵ķ���ʵ���У��轫�¶ȼƵ�ˮ�������Һ�������Կ���Һ���¶�
���ô������ۻ������ƵĻ�����
�ݲ���Һ���µ�pH���ò�����պȡ��Һ����ʪ���pH��ֽ�ϣ������ɫ������
����NaOH��Һ�еμ�Al2��SO4��3��Һ����Al2��SO4��3��Һ�еμ�NaOH��Һ����ͬ
�߿��ñ���̼��������Һ��ȥ������̼�л��е�������������
II. (6��)ijͬѧ�������ͼ��ʾ����ʵ��װ�ã�����װ��δ���������Ʊ�SO2������ʵ�������������仹ԭ�ԣ��Ʊ�SO2ʱѡ�õ��Լ�ΪCu��ŨH2SO4���ش��������⣺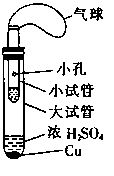
��1��д����ȡSO2�Ļ�ѧ����ʽ�� ��
��2���÷�Ӧ��ŨH2SO4���ֵĻ�ѧ������ ��
��3����ͬѧ����ʵ������������SO2�Ļ�ԭ�ԣ���ѡ�õ��Լ�Ϊ ��
A��˫��ˮ��H2O2�� B��Ʒ����Һ C�����Ը��������Һ
����SO2��ԭ�Ե��Թ��е�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ��ˮ��ѧ��һ��ѧ��һ�����Ի�ѧ�Ծ����������� ���ͣ������
����7��)ijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ�����ϵ��ʵ�飬ʵ��ʱ��ҩƷA��μ��뵽����B�У����������ʵ��ش����⣺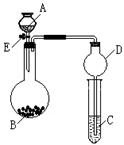
(1����AΪŨ��ˮ��BΪ�����ƣ�C��ʢ��AlCl3��Һ����������E���㹻��ʱ��۲쵽C�е�����Ϊ ��Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ ��
(2����AΪ30%��H2O2��Һ��BΪ�������̣�C��ʢ���ữ����FeCl2��Һ����������E��C�е�����Ϊ ��Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ ��
(3)��������װ�û�������֤SO2�Ļ�ѧ����, AΪ��Ũ���ᣬBΪ�������ƣ���C��ʢ��
��Һ����֤SO2��������; C��ʢ�� ��Һ����֤�仹ԭ��; ��C��ʢ�� ��Һ����֤��Ư���ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�Ͱ����и߶���ѧ�ڵ�һ���¿���ѧ�Ծ����������� ���ͣ�ʵ����
(13��)ijͬѧ�����������ͼ��ʵ��װ�������Եزⶨ��ʯ��̼���Ƶ�����������
��1����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ____________________________________________��װ��B��C��������_________________________________����ƿҪ��������ʯ��Ӧ������Ƥ��������Ϊ��_________________________________��
��2�����õ�ʯ��������̫����_________________________________��Ҳ����̫С������ _________________________________��������B���ݻ�Ϊ250 mL�������õ�ʯ������Ӧ��___________ g���ң�ѡ�0.03��0.60��1.00��1.50��2.00����
��3���ɷ�Һ©������ƿ��μ�ˮ�IJ���������________________________________��
��4��ʵ���в��������Ͳ��ˮ�����ΪV mL����ʯ������ΪW g�����ʯ��̼���Ƶ�����������___________%�������㵼���в�����ˮ�������б��͵�ˮ������Ҳ���Բ��ƣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧ���������� ��ѧ��ɳ�����չ��ϰ���������棩 ���ͣ�ʵ����
(10��)ijͬѧ�������ͼ��ʾװ�ý���ʯ�������ʵ�飬�ش������й����⣺

(1)ָ��ʵ��װ��������A��B��C��D�����ƣ�
A��________��B.________��C.________��D.________��
(2)ָ����ͬѧ����Ƶ�ʵ��װ���д��ڵĴ������������
(3)ʵ��װ�ø�������ν��������Լ�飿
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com