
��15�֣��ظ�����ǹ�ҵ������ʵ���ҵ���Ҫ����������ҵ�ϳ��ø�������Ҫ�ɷ�ΪFeO��Cr2O3��Ϊԭ������,ʵ����ģ�ҵ���ø�������K2Cr2O7����Ҫ�������£��漰����Ҫ��Ӧ�ǣ�6FeO��Cr2O3+24NaOH+7KClO3 12Na2CrO4+3Fe2O3
+7KCl+12H2O��
12Na2CrO4+3Fe2O3
+7KCl+12H2O��

�Իش��������⣺
��1���ڷ�Ӧ�����У���Na2CrO4���ɣ�ͬʱFe2O3ת��ΪNaFeO2������SiO2��Al2O3�봿�Ӧת��Ϊ�������Σ�д����������̼���Ʒ�Ӧ�Ļ�ѧ����ʽ��
��
��2�������۵�Ŀ����ʲô���ü�Ҫ������˵����
��
��3���������У��ữʱ��CrO42��ת��ΪCr2O72��,д��ƽ��ת�������ӷ���ʽ��
��
��4����ȡ�ظ��������2.5000g���250mL��Һ��ȡ��25.00mL�ڵ���ƿ�У�����10mL 2mol/LH2SO4�������⻯��(���Ļ�ԭ����ΪCr3+)�����ڰ���5min��Ȼ�����100mLˮ������3mL����ָʾ������0.1200mol/LNa2S2O3����Һ�ζ���
��I2+2S2O32��=2I��+S4O62����
���жϴﵽ�ζ��յ�������ǣ� ��
����ʵ���й���ȥNa2S2O3����Һ40.00mL,�����ò�Ʒ���ظ���صĴ��ȣ��������������������ʲ����뷴Ӧ�� ��������С�������λ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||

| pH | C��CrO42-��mol/L | C��HCrO4-��mol/L | C��Cr2O72-��mol/L | C��H2CrO4��mol/L |
| 4 | 0.0003 | 0.104 | 0.448 | 0 |
| 5 | 0.0033 | 0.103 | 0.447 | 0 |
| 6 | 0.0319 | 0.0999 | 0.437 | 0 |
| 7 | 0.2745 | 0.086 | 0.3195 | 0 |
| 8 | 0.902 | 0.0282 | 0.0347 | 0 |
| 9 | 0.996 | 0.0031 | 0.0004 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
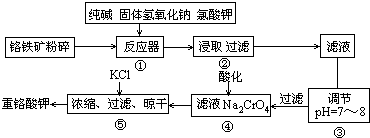
�ظ�����ǹ�ҵ������ʵ���ҵ���Ҫ����������ҵ�ϳ��ø�������Ҫ�ɷ�ΪFeO��Cr2O3��Ϊԭ������,ʵ����ģ�ҵ���ø�������K2Cr2O7����Ҫ�������£��漰����Ҫ��Ӧ�ǣ�6FeO��Cr2O3+24NaOH+7KClO3![]() 12Na2CrO4+3Fe2O3 +7KCl+12H2O
12Na2CrO4+3Fe2O3 +7KCl+12H2O

�Իش��������⣺
��1���ڷ�Ӧ�����У���Na2CrO4���ɣ�ͬʱFe2O3ת��ΪNaFeO2������SiO2��Al2O3�봿�Ӧת��Ϊ�������Σ�д����������̼���Ʒ�Ӧ�Ļ�ѧ����ʽ�� ��
��2�������۵�Ŀ����ʲô���ü�Ҫ������˵���� ��
��3���������У��ữʱ��CrO42��ת��ΪCr2O72��,д��ƽ��ת�������ӷ���ʽ�� ��
��4����ȡ�ظ��������2.5000g���250mL��Һ��ȡ��25.00mL�ڵ���ƿ�У�����10mL
2mol/LH2SO4�������⻯��(���Ļ�ԭ����ΪCr3+)�����ڰ���5min��Ȼ�����100mLˮ������3mL����ָʾ������0.1200mol/LNa2S2O3����Һ�ζ���I2+2S2O32��=2I��+S4O62����
���жϴﵽ�ζ��յ�������ǣ� ��
����ʵ���й���ȥNa2S2O3����Һ40.00mL,�����ò�Ʒ���ظ���صĴ��ȣ��������������������ʲ����뷴Ӧ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com