
����Ŀ���۴�����ϩ�����ϼ���Ӧ�ù㷺�������Ǹ��л���ĺϳ�·�ߣ�
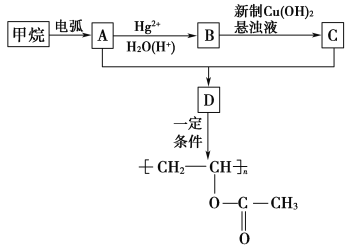
��ʾ���������ڵ绡������������Ȳ����
��CH3C��CH![]() CH3CH2CHO(δ��ƽ)��
CH3CH2CHO(δ��ƽ)��
��ش��������⣺
(1)����ϳ�A�Ļ�ѧ��Ӧ��ԭ��������Ϊ________��
(2)B�Ľṹ��ʽΪ________��
(3)B����C�ķ�Ӧ�г�����Cu(OH)2����Һ���Ҫ��������________��
(4)A��C��Ӧ����D�ķ�Ӧ������________��
(5)д����D����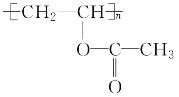 ��Ӧ�Ļ�ѧ����ʽ��________________________________��
��Ӧ�Ļ�ѧ����ʽ��________________________________��
(6)д����ʹ��ɫʯ����Һ����D������ͬ���칹��Ľṹ��ʽ��________________________________��
���𰸡�81.25%CH3CHO���ȼӳɷ�Ӧ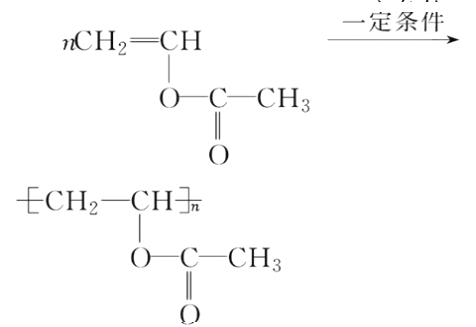 CH2===CHCH2COOH��CH3CH===CHCOOH��
CH2===CHCH2COOH��CH3CH===CHCOOH��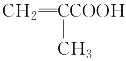
��������
��Ŀ�����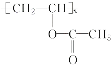 ��֪A��B��C���Ǻ���2��̼ԭ�ӵ��л�������ʾ��Ϣ��֪AΪ��Ȳ��BΪ��ȩ��CΪ���ᡣ�ݴ˷����ɵý��ۡ�
��֪A��B��C���Ǻ���2��̼ԭ�ӵ��л�������ʾ��Ϣ��֪AΪ��Ȳ��BΪ��ȩ��CΪ���ᡣ�ݴ˷����ɵý��ۡ�
(1)����������Ȳ�Ļ�ѧ��Ӧ����ʽΪ2CH4![]() CH��CH��3H2�����Ը÷�Ӧ��ԭ��������Ϊ26��32��100%��81.25%����2��B�Ľṹ��ʽΪ��CH3CHO (3)Bת��ΪCʱ��������������ͭ����Һ��������������ʱ��Ҫ���ȣ��ʴ�Ϊ�����ȣ� (4)��Ȳ�����ᷴӦ����
CH��CH��3H2�����Ը÷�Ӧ��ԭ��������Ϊ26��32��100%��81.25%����2��B�Ľṹ��ʽΪ��CH3CHO (3)Bת��ΪCʱ��������������ͭ����Һ��������������ʱ��Ҫ���ȣ��ʴ�Ϊ�����ȣ� (4)��Ȳ�����ᷴӦ����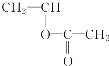 �����Ƿ�����̼̼�����ϵļӳɷ�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��(5)D����
�����Ƿ�����̼̼�����ϵļӳɷ�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��(5)D����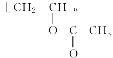 �ķ�ӦΪ�Ӿ۷�Ӧ������ʽΪ��
�ķ�ӦΪ�Ӿ۷�Ӧ������ʽΪ��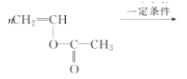
 ��(6)�ܹ�ʹ��ɫʯ����Һ����D��ͬ���칹���к����Ȼ����ʷ���������ͬ���칹�Ľṹ��ʽΪ��CH2=CHCH2COOH��CH3CH=CHCOOH��
��(6)�ܹ�ʹ��ɫʯ����Һ����D��ͬ���칹���к����Ȼ����ʷ���������ͬ���칹�Ľṹ��ʽΪ��CH2=CHCH2COOH��CH3CH=CHCOOH��![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״�����������л�����ԭ��֮һ����ҵ�Ͽ��ö�����̼��������Ӧ�������״���
(1)��֪��̬�״���ȼ����Ϊa kJ/mol��2H2(g)+O2(g)= 2H2O(g) ��H=-bkJ/mol��H2O(g)=H2O(l) ��H= -ckJ/mol�� ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)����H=_________��
CH3OH(g)+H2O(g)����H=_________��
(2)ij�¶��£���2 L�ܱ������У�����2.4 mol CO2��4.4 mol H2�������ϳɼ״��ķ�Ӧ����ü״������ʵ�����ʱ��ı仯ͼ������ͼ�е�����I����ǰ4������(CO2)=__________������1 minʱ���ı�ijһ��Ӧ����������I��Ϊ����II����ı������Ϊ___________�����¶��·�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ___________��
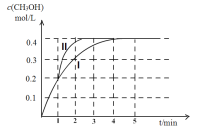
(3)�ں�ѹ�������£�����ѡ����˵��CO2+3H2![]() CH3OH+H2O��Ӧ�Ѵ�ƽ��״̬����______��
CH3OH+H2O��Ӧ�Ѵ�ƽ��״̬����______��
A������(H2): ����(CH3OH)=3:1
B�����������ܶȲ��ٱ仯
C���������ƽ��Ħ���������ٱ仯
D����Ӧ��H2O��CH3OH�����ʵ���Ũ��֮��Ϊ1:1���ұ�ֵ���ֲ���
(4)����һ�¶��·����ϳɼ״��ķ�Ӧ���ر�K����A�����г���1 mol CO2��4 mol H2����B�����г���1.2 mol CO2��4.8 mol H2���������ֱ���������Ӧ����֪��ʼʱ����A��B�������Ϊa L����Ӧ�ﵽƽ��ʱ����B�����Ϊ0.9a L��ά�������������䣬����Kһ��ʱ������´ﵽƽ�⣬����B�����Ϊ______L(�������¶ȵı仯��PΪ�����ɻ��������������ǻ�����Ħ����)��
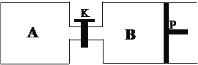
(5)һ�������¼״��ɽ�һ������ת��Ϊ���ᡣ�����£���amol/L�ļ�����bmol/L��NaOH��Һ�������ϣ���ϵ�д���c(Na+)=c(HCOO-)�����ú�a��b�Ĵ���ʽ��ʾ����ĵ��볣��Ϊ__________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��Ҫ����к��ȵIJⶨ��
��1��ʵ�����ϱ��д�С�����ձ�����ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β����������0.5 mol��L-1���ᡢ0.55 mol��L-1 NaOH��Һ��ʵ����ȱ�ٵIJ�����Ʒ��___________��___________��
��2��ʵ�����ܷ��û���ͭ˿��������滷�β��������___________������������������������
ԭ���� ��
��3�����Ǽ�¼��ʵ���������£�
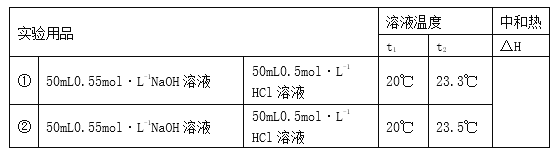
��֪��Q��cm��t2��t1������Ӧ����Һ�ı�����cΪ4.18 kJ����-1��kg-1�������ʵ��ܶȾ�Ϊ1 g��cm-3��
�ټ�������ϱ���H��_____________��
�ڸ���ʵ����д��NaOH��Һ��HCl��Һ��Ӧ���Ȼ�ѧ����ʽ�� ��
��4������KOH����NaOH���Բⶨ���__________��������������������Ӱ�죻���ô������HCl��ʵ�飬�Բⶨ���____________��������������������Ӱ�졣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
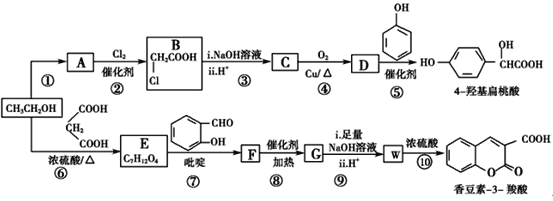
����Ŀ����ҵ�����Ҵ�Ϊԭ�Ͼ�һϵ�з�Ӧ���Եõ�4-�ǻ���������㶹��-3-���ᣬ���ߵĺϳ�·������(���ֲ��P����δ�г�)��
��֪����.RCOOR�䣫R��OH![]() RCOOR�士R��OH��
RCOOR�士R��OH��
��. 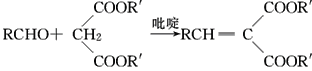 (R��R�䣬R���ʾ��ԭ�ӡ������)
(R��R�䣬R���ʾ��ԭ�ӡ������)
�ش��������⣺
(1)��Ӧ������ȡ����Ӧ����A�й����ŵ�������________
(2) 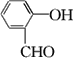 ��������________����Ӧ�ݵķ�Ӧ����Ϊ________��
��������________����Ӧ�ݵķ�Ӧ����Ϊ________��
(3)��Ӧ�Ļ�ѧ����ʽ��________________________________________��
(4)��֪G�����к���2����Ԫ���������й�˵����ȷ����________(����)��
a���˴Ź����ǿɲ��E��5�����͵���ԭ��
b�������ǿɼ��F������ʺɱȵ�ֵΪ236
c��G����ʽΪC12H10O4
d��������W�ܷ����Ӿ۷�Ӧ�õ����߷��ӻ�����
(5)ij���㻯����Q��4-�ǻ��������ͬ���칹�壬���������������ٱ�����ֻ��3��ȡ���������ܷ���ˮ�ⷴӦ��������Ӧ����1 mol Q���������3 mol NaOH��Q����________��(���������칹)
(6)��ϸ�۲����Ҵ��ϳ��㶹��-3-����Ĺ��̣���������Ϣ�����Ҵ������������ʵ����ı�Ϊ________ʱ��ֻ��3��������ɺϳ�·�ߡ���д���ϳ�·��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ֱ���������������Ļ��ᴦ����������ϴ��Һ�к���Fe3+��Ni2+��NO3-��F-��+6�۸��ĺ���������ӵȣ���ͼ���ۺ����ø���ϴ��Һ�Ĺ������̣�
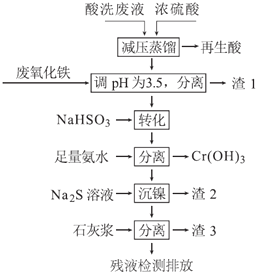
��֪��
�ٽ������ӿ�ʼ�����ͳ�����ȫʱ��pH��
Fe3+ | Ni2+ | Cr3+ | |
��ʼ���� | 1.5 | 6.7 | 4.0 |
������ȫ | 3.4 | 9.5 | 6.9 |
��Ni2+��������ˮ�ķ�ӦΪ��Ni2++6NH3[Ni��NH3��6]2+
��1���������к���______����ȡ��ѹ�����ԭ����______���û�ѧ����ʽ��ʾ����
��2�����÷�����������Ҫ�ɷ�ΪFe2O3�������ռ����pH�ĺô���______��
��3����д����ת����ʱNaHSO3��Cr2O72-������Ӧ�����ӷ�Ӧ����ʽ��______��
��4����֪[Ni��NH3��6]2+Ϊ�ѵ����������ӣ������������ӷ���ʽΪ��______��
��5������3����Ҫ�ɷ�ΪCa��OH��2��_________________________��
��6������⣬���IJ�Һ��c��Ca2+��=0.004molL-1�����Һ��F-Ũ��Ϊ______mgL-1��[��֪Ksp��CaF2��=4��10-11mol3L-3.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���ѧ����]MΪ�ϳɸ߷��Ӳ��ϵ��м��壬�Է�����A�Ʊ�M�߷��ӻ�����N��һ�ֺϳ�·�����£�

��֪��
��ش��������⣺
��1��C�Ļ�ѧ����Ϊ________��
��2��A��B��H��M�ķ�Ӧ���ͷֱ�Ϊ________��________��
��3��F�����������ŵ�����Ϊ________��G�Ľṹ��ʽΪ________��
��4���Լ�1Ϊ________��
��5��D��N�Ļ�ѧ����ʽΪ________��
��6��QΪH��ͬ���칹�壬ͬʱ��������������Q�Ľṹ��ʽΪ________��
�ٱ�������������ȡ����������������������״�ṹ
�������Ȼ�����Һ������ɫ��Ӧ��1 mol Q�������3 molNaOH
�ۺ˴Ź���������5��壬�����֮��Ϊ6��2��2��1��1
��7�����������ϳ�·�ߺ���Ϣ���Լ�ȩ����ȩΪ��ʼԭ�ϣ����Լ���ѡ��������Ʊ��۱�ϩ�ᣨ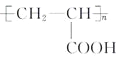 ���ĺϳ�·�ߣ�________��
���ĺϳ�·�ߣ�________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na2O2������Ư���ͺ�������еĹ�������
��1��ijѧϰС�鷢�֣���ʢ��Na2O2���Թ��м�������ˮ�����������������ݣ���������ʧ�������е���1~2�η�̪��Һ����Һ��죻���Թ���������ɫ�ܿ���ȥ����ʱ�����Թ��м�������MnO2��ĩ���������ݲ�����
��ʹ��̪��Һ�������Ϊ____________________________________����ɫ��ȥ�Ŀ���ԭ����____________________________________________��
�ڼ���MnO2��Ӧ�Ļ�ѧ����ʽΪ_______________________________��
��2��Na2O2��ǿ�����ԣ�H2���л�ԭ�ԣ���ͬѧ����Na2O2��H2�ܷ�Ӧ��Ϊ����֤�˲��룬��С��ͬѧ��������ʵ�飬ʵ�鲽����������¡�
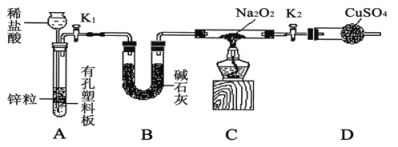
����1������ͼ��װ������ͼ�мг�����ʡ�ԣ�����������ԣ�װ��ҩƷ��
����2����K1��K2����������������װ��Na2O2��Ӳ�ʲ����ܣ�һ��ʱ���û���κ�����
����3������H2�Ĵ��Ⱥ�ʼ���ȣ��۲쵽Ӳ�ʲ�������Na2O2��ʼ�ۻ�������ɫ�ķ�ĩ����˰�ɫ���壬�����������ͭδ����ɫ��
����4����Ӧ��ȥ�ƾ��ƣ���Ӳ�ʲ�������ȴ��ر�K1��
��ʢװϡ�������������___________��Bװ�õ�������______________��
�ڱ�������������ȵ�ԭ����_________________��
������װ��D��Ŀ����___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ǽ�������A����ͼ��ʾ�Ĺ���ת��Ϊ������D����֪DΪǿ�ᣬ��ش��������⣺
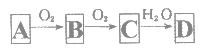
��1����A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ���塣
��D�Ļ�ѧʽ��_____��
���ڹ�ҵ�����У�B����Ĵ����ŷű���ˮ���պ���γ��������Ⱦ������д���γ�����Ļ�ѧ����ʽ______����ҵ�ϳ���ú�м���ʯ��ʯ������B����Ի�������Ⱦ���䷴Ӧ�Ļ�ѧ����ʽΪ_____��
��2����A�ڳ�����Ϊ���壬C�Ǻ���ɫ�����塣
��Aת��ΪB�Ļ�ѧ����ʽ��____��
��D��ϡ��Һ�ڳ����¿���ͭ��Ӧ������B���壬��д���÷�Ӧ�����ӷ���ʽ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������̼���������潡����������С������һ�����������й��ڵ������������˵����ȷ����( )
A.���쳵�ָ�Ȧ�IJ����ǺϽ�B.���Ͻ�����ij��ܽϸ��������
C.������̥�õ����й̶����۵�D.�����������л��������ڹ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com