
���÷Ͼ�п��Ƥ�Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ�����ͼ���£�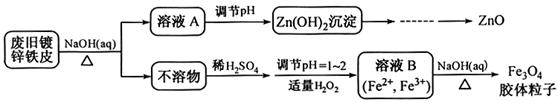
��֪��Zn���������������Al����������������ơ���ش��������⣺
��1����NaOH��Һ�����Ͼ�п��Ƥ�������� ��
| A��ȥ������ | B���ܽ��п�� | C��ȥ������ | D���ۻ� |
��1��A��B
��2�����ˡ�ϴ�ӡ�����
��3��N2�����£���ֹFe2+������
��4�����ܣ���������̫С������ʱ��������ֽ
��5��0.7350 �ۢ�
��6��ƫ��
������1������Zn���������������Al����������������ƣ�ZnҲ�ܺ�����������Һ��Ӧ������������Һ���ܽ��п���ȥ���������á�
������ҺA��pH�ɲ���Zn(OH)2���������˾Ϳ��Եõ�������п�������������գ�������п�ֽ�� ��ZnO��
��3������ͨ��N2����ֹFe2+��������
Fe3O4��������������ֽ�����Բ����ù��˵ķ���ʵ�ֹ�Һ���롣
��5��m(K2Cr2O7)="0.01000" mol��L��1��0.250 L��294.0 g��mol��1="0.7350" g��������ƽ���ڳ��������������ձ������ܽ���壬���������ڽ������������������ƿ����������Һ����ͷ�ι����ڼ�ˮ���ݡ��ò���������Ϊ��Ͳ����Һ�ܡ�
��6������ζ�ǰװ��K2Cr2O7����Һ�ĵζ��ܼ��첿�������ݣ���������ɶ���ƫС���ζ�������������ʧ���������������������Զ�������K2Cr2O7����Һ���ƫ�ⶨ�����ƫ��
�����㶨λ�������ԡ��Ͼɶ�п��Ƥ���Ʊ�����Fe3O4�������Ӽ�������ZnO��Ϊ���壬����ʵ����������ͼ��ܡ��漰����Һ�����Ƽ��ζ�����������������Ҫȡ���ڡ�ʵ�黯ѧ��ģ���С���п��Ƥ�Ʋ��ȵIJⶨ���������ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ȫ����ͨ�ߵ�ѧУ����ͳһ���������ۺ��������Ի�ѧ���㽭�������棩 ���ͣ�ʵ����
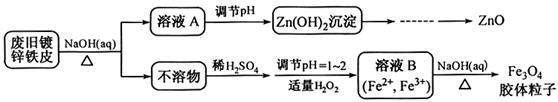
���÷Ͼ�п��Ƥ�Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ�����ͼ���£�
��֪��Zn���������������Al����������������ơ���ش��������⣺
��1����NaOH��Һ�����Ͼ�п��Ƥ�������� ��
A��ȥ������ B���ܽ��п�� C��ȥ������ D���ۻ�
��2��������ҺA��pH�ɲ���Zn(OH)2������Ϊ�Ƶ�ZnO���������������� ��
��3������ҺB�Ƶ�Fe3O4�������ӵĹ����У������ͨ��N2��ԭ���� ��
��4��Fe3O4���������ܷ��ü�ѹ���˷�ʵ�ֹ�Һ���룿 ����ܡ����ܡ����������� ��
��5�����ظ���ط���һ��������ԭ�ζ������ɲⶨ����Fe3O4�еĶ�������������������Ũ��Ϊ0.01000 mol��L��1��K2Cr2O7����Һ250 mL��Ӧȷ��ȡ g K2Cr2O7(����4λ��Ч���֣���֪M(K2Cr2O7)��294.0 g��mol��1)��
���Ƹñ���Һʱ�����������в���Ҫ�õ����� �����ñ�ű�ʾ����
�ٵ�����ƽ ���ձ� ����Ͳ �ܲ����� ������ƿ ��ͷ�ι� ����Һ��
��6���ζ������У�����ζ�ǰװ��K2Cr2O7����Һ�ĵζ��ܼ��첿�������ݣ����ζ�������������ʧ����ⶨ����� ���ƫ����ƫС�����䡱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߿���ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com