
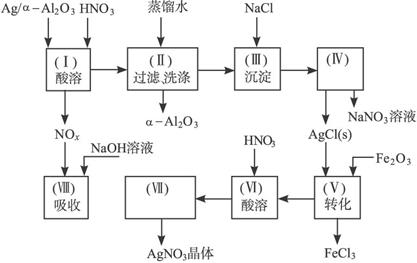
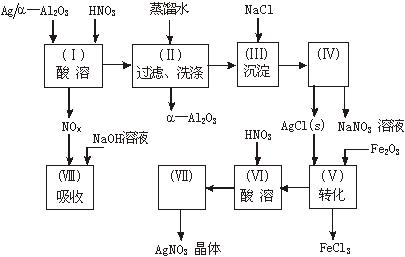
ͼ15-18
�Ķ�����ʵ�����̣����������գ�
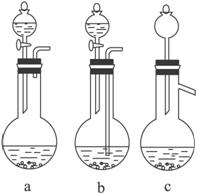
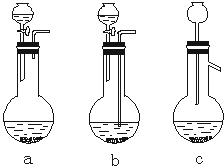
��1��Ag/��Al2O3�����ܽ�Ӧ��ѡ��װ��____________________________��ѡ��a��b��c����
��2����ʵ������������������ˮ��������ˮ����ϴ�ӣ����ᷢ����ѧ��Ӧ�����ӷ���ʽΪ_________________________________��
��3��ʵ��������������貣������Ϊ_________________����д���֣���
��4��ʵ�������������AgNO3��Һ���AgNO3������Ҫ���е�ʵ���������Ϊ��________��
a.���� b.���� c.���� d.���� e.��ȴ�ᾧ
��5����֪��NO+NO2+2NaOH====2NaNO2+H2O��
2NO2+2NaOH====NaNO3+NaNO2+H2O��
NO��NO2�Ļ���������ɿɱ�ʾΪNOx���û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵΪ________________��
a.x��1.5 b.x=1.2 c.x��1.5
��6����֪Ag/��Al2O3��Ag������������������Ag�Ļ����ʣ�������֪����ʵ������Ϊ_____��__________��
������������һ���ۺ��Խ�ǿ����Ŀ������ҪŪ��ʵ�����̵�Ŀ�ĺͷ�Ӧԭ����Ȼ�������ȷ��𡣣�1����Ag����HNO3��Al2O3���ܣ���Ag����HNO3ʱ��ų�NOx��Ӧѡװ��a����2����������ˮ����Cl-���������Ag++Cl-====AgCl������3��ʵ������������ǹ��˳�AgCl������������˲��������������Ҫ����Ϊ�ձ�����������©����ע�⿴��Ҫ�ش�������������4��Ҫ��AgNO3��Һ�л��AgNO3���壬�ر�ע�ⲻ�����ա���AgNO3�ֽ⡣��5����������������ʽ������NO���ܱ��������ն�NO2�ɱ��������ա���ô������屻��ȫ����ʱ��x��1.5����6��Ҫ����Ag�Ļ����ʱ���֪����������������������Ag�������������Ag������������Ҫ֪���������AgNO3��������
�𰸣���1��a ��2��Ag++Cl-====AgCl�� (3)©�����ձ��������� ��4��bed ��5��c
��6������������ AgNO3������


 С�����ϵ�д�
С�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ϻ��߿����� ���ͣ�ʵ����

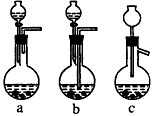
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ag/����Al2O3��ʯ�ͻ�ѧ��ҵ��һ����Ҫ����������Ag������ã�����Al2O3�������Ҳ��������ᣬ�ô����Ļ���ʵ������ͼ��ʾ�����е�ת����ӦΪ��6AgCl��Fe2O3 = 3Ag2O��2FeCl3

�Ķ�����ʵ�����̣����������գ�
��1��Ag/����Al2O3�����ܽ�Ӧ��ѡ��װ�� ��ѡ��a��b��c����
��2����ʵ������������������ˮ��������ˮ����ϴ�ӣ����ᷢ����ѧ��Ӧ�����ӷ���ʽ ��
��3��ʵ��������������貣������Ϊ _����д���֣���
��4��ʵ�������������AgNO3��Һ���AgNO3������Ҫ���е�ʵ���������Ϊ___________��
a. ���� b. ���� c. ���� d . ���� e. ��ȴ�ᾧ
��5����֪��NO��NO2��2NaOH��2NaNO2��H2O��
2NO2��2NaOH��NaNO3��NaNO2��H2O
NO��NO2�Ļ���������ɿɱ�ʾΪNOx���û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵΪ______��a. x��1.5 b. x��1.2 c. x��1.5
��6����֪Ag/����Al2O3��Ag������������������Ag�Ļ����ʣ�������֪����ʵ������Ϊ�� ____��
��7��������������AgNO3������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ķ�����ʵ�����̣����������գ�
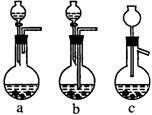
��1��Ag/����Al2O3�����ܽ�Ӧ��ѡ��װ��������ѡ��a��b��c����
��2����ʵ������������������ˮ��������ˮ����ϴ�ӣ����ᷢ����ѧ��Ӧ�����ӷ���ʽ
����������������������������������
��3��ʵ��������������貣������Ϊ����������������д���֣���
��4��ʵ�������������AgNO3��Һ���AgNO3������Ҫ���е�ʵ���������Ϊ������������������ѡ�۷֣���
��a������b�������� ��c�����ա� ��d�����ˡ� ��e����ȴ�ᾧ
��5����֪��NO+NO2+2NaOH![]() 2NaNO2+H2O��
2NaNO2+H2O��
2NO2+2NaOH![]() NaNO3+NaNO2+H2O
NaNO3+NaNO2+H2O
NO��NO2�Ļ���������ɿɱ�ʾΪNOx���û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵΪ
��������������
��a��x��1.5�� ��b��x=1.2�� ��c��x��1.5
��6����֪Ag/����Al2O3��Ag������������������Ag�Ļ����ʣ�������֪����ʵ������Ϊ��������������
��������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com