��2012?������ģ��A��B��C��D����Ԫ��ԭ�ӵĺ˵������������С��20�����䵥�ʼ���Ӧ�Ļ���������ͼ��ϵ��
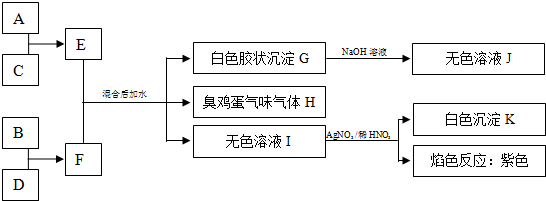
��1��Ԫ��A�����ڱ��е�λ��Ϊ
�������ڵڢ�A��
�������ڵڢ�A��
��
��2��д��F�ĵ���ʽ
��
��3������H�������Na
2CO
3��Һ���գ�����������ʽ�Σ��÷�Ӧ�����ӷ���ʽΪ��
H2S+CO32-�THS-+HCO3-
H2S+CO32-�THS-+HCO3-
��
��4����E��ˮ��Һ�������ɲ����գ����յõ��Ĺ���Ϊ
Al2O3
Al2O3
��ԭ��Ϊ���û�ѧ����ʽ��ʾ��
AlCl3+3H2O?Al��OH��3+3HCl
AlCl3+3H2O?Al��OH��3+3HCl
��
��
��5�������£���F��ˮ��Һ�м���������Ũ�ȵ������������Һ��pH��7�������Һ�и������ӵ�Ũ����С�����˳��Ϊ
c��S2-����c��H+����c��OH-����c��HS-����c��Cl-����c��K+��
c��S2-����c��H+����c��OH-����c��HS-����c��Cl-����c��K+��
��




 ��
�� ��
��


 Ca2++2OH-������������Һ����ʹCa��OH��2���ٵ��ǣ�������
Ca2++2OH-������������Һ����ʹCa��OH��2���ٵ��ǣ�������