�������л���A����Է�������Ϊ164��8.2g�л���A�����ʵ���=
=0.05mol����ȫȼ�����ɱ�״����11.2LCO
2��5.4gH
2O���������̼Ϊ
=0.5mol��ˮΪ
=0.3mol�������A��N��C��=
=10��N��H��=
=12����N��O��=
=2������A�ķ���ʽ��C
10H
12O
2��
F��һ�������ºϳɸ߷�����֬��

����Ӧ�Ƿ���������Ӧ����FΪ
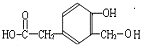
��A������Ϣ��Ӧ����B��C��B�ܱ�����������ͭ��������D����B����-CHO��D����-COOH��D������Ϣ���еڶ�����Ӧ����F����D����-OCH
3�����F�Ľṹ��֪��DΪ
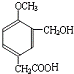
����BΪ
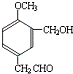
���л���E��Һ�ε�������ˮ�У��д�����ɫ�������ɣ���E���з��ǻ������C��E��Ӧ����ṹ��H��C��E��Ӧ����ṹ��֪��CΪHCHO��EΪ
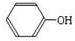
������������A�ĽṹΪ
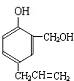
���ݴ˽��
����⣺�л���A����Է�������Ϊ164��8.2g�л���A�����ʵ���=
=0.05mol����ȫȼ�����ɱ�״����11.2LCO
2��5.4gH
2O���������̼Ϊ
=0.5mol��ˮΪ
=0.3mol�������A��N��C��=
=10��N��H��=
=12����N��O��=
=2������A�ķ���ʽ��C
10H
12O
2��
F��һ�������ºϳɸ߷�����֬��

����Ӧ�Ƿ���������Ӧ����FΪ

��A������Ϣ��Ӧ����B��C��B�ܱ�����������ͭ��������D����B����-CHO��D����-COOH��D������Ϣ���еڶ�����Ӧ����F����D����-OCH
3�����F�Ľṹ��֪��DΪ

����BΪ

���л���E��Һ�ε�������ˮ�У��д�����ɫ�������ɣ���E���з��ǻ������C��E��Ӧ����ṹ��H��C��E��Ӧ����ṹ��֪��CΪHCHO��EΪ

������������A�ĽṹΪ

��
��1��������������֪��A�ķ���ʽΪ��C
10H
12O
2��
�ʴ�Ϊ��C
10H
12O
2��
��2����Ӧ�н�ȩ������Ϊ�Ȼ���Ҳ�����������ǻ�����Ӧ��ʼ���ǻ�������Ӧ����ȩ������Ϊ�Ȼ���D��F�ֲ������ǻ����ʲ�����Ŀ���DZ������ǻ���
�ʴ�Ϊ���������ǻ���
��3��E�DZ��ӣ����������ŵ��������ǻ��������ʽṹ��֪��H���ϳ������ķ�Ӧ�г����ɸ߾����⣬��ԭС�����������ɣ��������۷�Ӧ��
�ʴ�Ϊ���ǻ������۷�Ӧ��
��4��B��D�Ļ�ѧ����ʽ��

��
�ʴ�Ϊ��

��
��5��F���߷�����֬�Ļ�ѧ����ʽ��

��
�ʴ�Ϊ��

��
��6��F��ͬ���칹��X���������ص㣺��F������ͬ�Ĺ����ţ�1mol X �������Na��NaOH��NaHCO
3�����ʵ����ֱ�Ϊ3��2��1����X�к���1��-COOH��1�����ǻ���1�����ǻ���������������ȡ�����������Ϊ-OH��-CH
2CH��OH��COOH��-CH��OH��CH
2COOH������㶹�صĽṹ��ʽ

����֪X������ȡ����������λλ�ã���X�Ľṹ��ʽΪ��

��
X����ȡ����Ӧ�����㶹�ᣬ��X�����㶹��Ļ�ѧ����ʽ�ǣ�

��
�ʴ�Ϊ��

��

��

 ����ij�ϳ�������
����ij�ϳ�������  ����ԭ��֮һ����صĺϳ�·������ͼ��ʾ��ijЩ����������ȥ����
����ԭ��֮һ����صĺϳ�·������ͼ��ʾ��ijЩ����������ȥ����
 ��R1��R2��R3����������
��R1��R2��R3����������





 ��X�Ľṹ��ʽΪ
��X�Ľṹ��ʽΪ



 ����Ӧ�Ƿ���������Ӧ����FΪ
����Ӧ�Ƿ���������Ӧ����FΪ ��A������Ϣ��Ӧ����B��C��B�ܱ�����������ͭ��������D����B����-CHO��D����-COOH��D������Ϣ���еڶ�����Ӧ����F����D����-OCH3�����F�Ľṹ��֪��DΪ
��A������Ϣ��Ӧ����B��C��B�ܱ�����������ͭ��������D����B����-CHO��D����-COOH��D������Ϣ���еڶ�����Ӧ����F����D����-OCH3�����F�Ľṹ��֪��DΪ ����BΪ
����BΪ ���л���E��Һ�ε�������ˮ�У��д�����ɫ�������ɣ���E���з��ǻ������C��E��Ӧ����ṹ��H��C��E��Ӧ����ṹ��֪��CΪHCHO��EΪ
���л���E��Һ�ε�������ˮ�У��д�����ɫ�������ɣ���E���з��ǻ������C��E��Ӧ����ṹ��H��C��E��Ӧ����ṹ��֪��CΪHCHO��EΪ ������������A�ĽṹΪ
������������A�ĽṹΪ ���ݴ˽��
���ݴ˽�� ����Ӧ�Ƿ���������Ӧ����FΪ
����Ӧ�Ƿ���������Ӧ����FΪ ��A������Ϣ��Ӧ����B��C��B�ܱ�����������ͭ��������D����B����-CHO��D����-COOH��D������Ϣ���еڶ�����Ӧ����F����D����-OCH3�����F�Ľṹ��֪��DΪ
��A������Ϣ��Ӧ����B��C��B�ܱ�����������ͭ��������D����B����-CHO��D����-COOH��D������Ϣ���еڶ�����Ӧ����F����D����-OCH3�����F�Ľṹ��֪��DΪ ����BΪ
����BΪ ���л���E��Һ�ε�������ˮ�У��д�����ɫ�������ɣ���E���з��ǻ������C��E��Ӧ����ṹ��H��C��E��Ӧ����ṹ��֪��CΪHCHO��EΪ
���л���E��Һ�ε�������ˮ�У��д�����ɫ�������ɣ���E���з��ǻ������C��E��Ӧ����ṹ��H��C��E��Ӧ����ṹ��֪��CΪHCHO��EΪ ������������A�ĽṹΪ
������������A�ĽṹΪ ��
�� ��
�� ��
�� ��
�� ��
�� ����֪X������ȡ����������λλ�ã���X�Ľṹ��ʽΪ��
����֪X������ȡ����������λλ�ã���X�Ľṹ��ʽΪ�� ��
�� ��
�� ��
�� ��
��

