
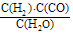 ��������Ӧ��Ӧ�Ļ�ѧ����ʽΪ_______________________��
��������Ӧ��Ӧ�Ļ�ѧ����ʽΪ_______________________��  CH3OH(g) ��H1����90.7kJ/mol
CH3OH(g) ��H1����90.7kJ/mol CH3OCH3(g) ��H2O(g) ��H2����23.5kJ/mol
CH3OCH3(g) ��H2O(g) ��H2����23.5kJ/mol CO2(g)��H2(g) ��H3����41.2kJ/mol
CO2(g)��H2(g) ��H3����41.2kJ/mol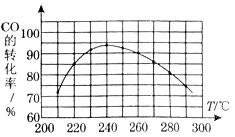
 CO��g ��+H2��g��
CO��g ��+H2��g�� CH3OCH3(g)��CO2(g) ��H����246.1 kJ/mol����
CH3OCH3(g)��CO2(g) ��H����246.1 kJ/mol����

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�긣��ʡ�ĵ���У������ѧ�ڵ������¿���ѧ�Ծ��������棩 ���ͣ������
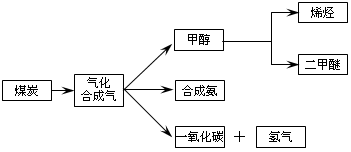
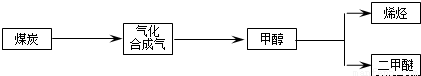
����ú��ƶ�͡����������ҹ���Դ��չ���ٵ���״��������Դ��������ţ���չ��ú���������ҹ���Դ�ṹ�ĵ���������Ҫ���塣��ͼ��ú������ҵ��֮һ��

���ྻú�������о����������൱�ձ飬������Աͨ�������ú������¯�н�����������ˮ�����ķ���������������ֵ�ܸߵ�ú̿�ϳ���������Ҫ�ɷ���CO��H2��CO��H2����Ϊ��Դ�ͻ���ԭ�ϣ�Ӧ��ʮ�ֹ㷺��
��1����֪��C(s)+O2(g)=CO2(g) ��H1����393.5 kJ��mol�C1 ��
C(s)+H2O(g)=CO(g)+H2(g) ��H2����131.3 kJ��mol�C1 ��
��ӦCO(g)+H2(g) +O2(g)= H2O(g)+CO2(g)����H= _________kJ��mol�C1���ڱ�״���£�33.6 L��ú̿�ϳ���(��ȫ��ΪCO��H2)��������ȫ��Ӧ����CO2��H2O����Ӧ��ת��______mole����
��2����һ���ݵ��ܱ������У���CO��H2�ϳɼ״���CO(g)+2H2(g) CH3OH(g)
CH3OH(g)
������������˵��������Ӧ�Ѵﵽƽ��״̬����_______
a����ϵѹǿ���ֲ���
b���ܱ�������CO��H2��CH3OH(g)3�����干��
c��CH3OH��H2���ʵ���֮��Ϊ1:2
d��ÿ����1 mol CO��ͬʱ����2molH2
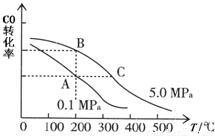
��CO��ƽ��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��

A��B�����ƽ�ⳣ��_____(�ǰ�ߡ��������ߡ���һ����)�� �ﵽA��C�����ƽ��״̬�����ʱ��tA tC(����ڡ�����С�ڡ����ڡ�)��
�ڲ��ı䷴Ӧ������������£�Ϊ���CO��ת���ʿɲ�ȡ�Ĵ�ʩ��_____________(������㼴��)��
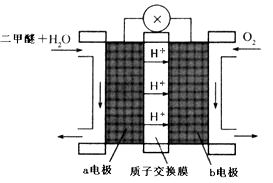
��3�������¶�650���������ȼ�ϵ�أ�����ú̿��(CO��H2)������ȼ����������CO2�Ļ������Ϊ����ȼ������һ��������Li2CO3��Na2CO3���۵�����������ʣ��Խ�����(ȼ�ϼ�)Ϊ�����Ƴɵġ������ĵ缫��ӦʽΪ��CO + H2��4e�� + 2CO32��= 3CO2+H2O����õ�ص�������ӦʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�걱������ͷ�����߿���ѧһģ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com