
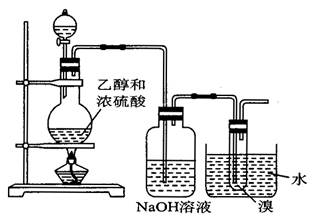
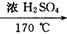 CH2�TCH2�� + H2O�����ڵĸ���Ӧ�Т�ŨH2SO4ʹ�Ҵ���ˮ̼����������2H2SO4 ( Ũ ) + C�T�T2SO2�� + CO2�� + 2H2O��Ӧ����CH3CH2OH + HO��CH2CH3
CH2�TCH2�� + H2O�����ڵĸ���Ӧ�Т�ŨH2SO4ʹ�Ҵ���ˮ̼����������2H2SO4 ( Ũ ) + C�T�T2SO2�� + CO2�� + 2H2O��Ӧ����CH3CH2OH + HO��CH2CH3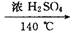 CH3CH2��O��CH2CH3 ( ���� ) + H2O�����Ա�������¶���170 ��,�����������ϩ���ʵ���֤��CH2�TCH2
CH3CH2��O��CH2CH3 ( ���� ) + H2O�����Ա�������¶���170 ��,�����������ϩ���ʵ���֤��CH2�TCH2 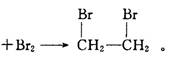


 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������֭�к��зḻ�������Ǻ��ǣ���������ԭ�Ǽ������������ |
| B������Ҷ�ϱ�Ƥ����Ҷ���壬���������۲�Ҷ����IJ��� |
| C���ø߱������Ϳ��Թ۲쵽��ǻ��Ƥϸ���е������壬�����õ������� |
| D�������¹۲�������˿�������õIJ��ϱ���ʼ�մ��ڻ״̬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 Z+W
Z+W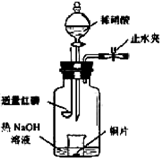
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
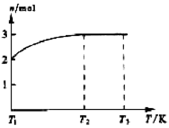
 2NH3(g) ��H����92.4 kJ/mol
2NH3(g) ��H����92.4 kJ/mol| A������������������ʱ��˵���÷�Ӧһ���ﵽƽ��״̬ |
| B�����������������ԭ���Ķ�������(��)��С����(��)����ƽ�������ƶ� |
| C�������������N2��ת���ʣ��������̴ﵽƽ������ʱ�䣬�������Ч�� |
| D�����ܱ������з���1 mol N2��3 mol H2���з�Ӧ����÷�Ӧ�ų�������С��92.4 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
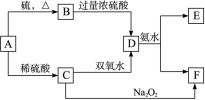
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
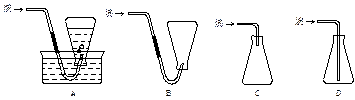
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ���ʵ�� | Ԥ��Ŀ�� |
| A | �ѵ�����������ͬ�ִ���ʯ���е�һ���гɷ�ĩ����ͬ�¶��·ֱ�������ͬŨ�ȵ����ᷴӦ���۲�ų���������� | ��֤������Ի�ѧ��Ӧ���ʵ�Ӱ�졣 |
| B | ��װ����ɫ��ͬ��NO2��N2O4��������С�Թܣ��ܷ⣩�ֱ������ˮ����ˮ�У��۲��Թ���������ɫ�仯�� | ��֤�¶ȶԻ�ѧƽ���Ӱ�졣 |
| C | ֱ�ӽ��������ͬ��������þ��Ͷ��ͬ�¶ȵ��з�̪����ˮ�У��۲�������ݵ����ʼ���Һ����ɫ�仯�� | �Ƚ�ͬ���ڽ���Ԫ�صĽ�����ǿ���� |
| D | ��װ��ͬ�ֻ��ǵı�����Һ�������Թ��У��ֱ����Na2CO3��NaHCO3���壬���ȣ��۲���Һ�Ƿ����塣 | �Ƚ�̼�ᡢ���Ӻ�̼���������Ե�ǿ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com