
����Ŀ���״�(CH3OH)������Ϊ��ɫҺ�壬��Ӧ�ù㷺�Ļ���ԭ�Ϻ�ǰ���ֹ۵�ȼ�ϡ�
(1)��֪��CH4(g)��H2O(g)CO(g)��3H2(g) H����206.0kJ/mol��1
CH4(g)��H2O(g)CH3OH(g)��H2(g) H����77.0kJ/mol��1
��CO��H2��Ӧ����CH3OH(g)���Ȼ�ѧ����ʽ��______________________��
(2)�״������ںϳ�3��5-�����������ӣ���Ӧ���£�
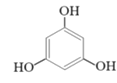 +2CH3OH
+2CH3OH![]()
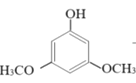 +2H2O
+2H2O
��Ӧ�������ȷ�����״����ټ������ѣ�����õ��л���(�������Ȼ���)����ϴ�ӣ�Ȼ������ᴿ�õ�����״���3��5-�����������ӵIJ����������ʼ�����
���� | �е�/�� | �۵�/�� | �ܽ��� |
�״� | 64.7 | ��97.8 | ������ˮ |
3��5-������������ | 172~175 | 33~36 | �����ڼ״������ѣ�����ˮ |
�ٷ�����״��IJ�����______________________(����ĸ���)��
a������ b����Һ c���ᾧ
��ϴ��ʱ�������ڳ�ȥ�л����е��Ȼ�����Լ���______________________(����ĸ���)��
a��Na2CO3��Һ b��NaHCO3��Һ c��NaOH��Һ
(3)�״�������ʵ�����Ʊ���Ȳ�����(CH![]() C��COOCH3���е�Ϊ103��105��)��
C��COOCH3���е�Ϊ103��105��)��
��ӦΪ��CH��C��COOH+CH3OH![]() CH��C��COOCH3+H2O
CH��C��COOCH3+H2O
ʵ�鲽�����£�
����1���ڷ�Ӧƿ�У�����14g��Ȳ�ᡢ50mL�״���2 mLŨ���ᣬ���裬���Ȼ���һ��ʱ�䡣
����2�����������ļ״�(װ����ͼ��ʾ)��

����3����ӦҺ��ȴ�������ñ���NaCl��Һ��5��Na2CO3��Һ��ˮϴ�ӡ�������л��ࡣ
����4���л��ྭ��ˮNa2SO4������ˡ����ñ�Ȳ�������
������A��������______________��������ƿ�м������Ƭ��Ŀ����_______________��
�ڲ���3�У���5%Na2CO3��Һϴ�ӣ���Ҫ��ȥ��������______________________��������л���IJ�������Ϊ_____________________��
�۲���4�У�����ʱ������ˮԡ���ȵ�ԭ����______________________��
���𰸡�CO(g)��2H2(g)CH3OH(g) H����129.0kJ/mol a b ������ ��ֹ���� ��Ȳ�� ��Һ ��Ȳ������ķе��ˮ�ĸ�
��������
(1)��֪��CH4(g)��H2O(g)CO(g)��3H2(g) H����206.0kJ/mol��1 ��
CH4(g)��H2O(g)CH3OH(g)��H2(g) H����77.0kJ/mol��1 ��
���ø�˹���ɣ�����-�٣�����CO��H2��Ӧ����CH3OH(g)���Ȼ�ѧ����ʽ��
(2)�ټ״��������л���ķе����ϴ��ɴ�ȷ��������״��IJ���������
��ϴ��ʱ�������ڳ�ȥ�л����е��Ȼ�����Լ���Ӧ������HCl��������ǻ�����Ӧ��
(3)������A�������������ܣ�������ƿ�м������Ƭ������Һ���ܹ�ƽ�ȵط��ڡ�
�ڲ���3�У���5%Na2CO3��Һϴ�ӣ���Ҫ��ȥ�����ʳ����ԣ��л�����Na2CO3��Һ�����ܣ��ӷֲ��Һ���з�����л��࣬��ȷ����������ơ�
�۲���4�У�����ʱ������ˮԡ���ȵ�ԭ�ӷе㿼�ǡ�
(1)��֪��CH4(g)��H2O(g)CO(g)��3H2(g) H����206.0kJ/mol��1 ��
CH4(g)��H2O(g)CH3OH(g)��H2(g) H����77.0kJ/mol��1 ��
���ø�˹���ɣ�����-�٣�����CO��H2��Ӧ����CH3OH(g)���Ȼ�ѧ����ʽΪCO(g)��2H2(g)CH3OH(g) H����129.0kJ/mol����Ϊ��CO(g)��2H2(g)CH3OH(g) H����129.0kJ/mol��
(2)�ټ״��������л���ķе����ϴ��������״��IJ�������Ϊ����ѡa����Ϊ��a��
��ϴ��ʱ�������ڳ�ȥ�л������Ȼ�����Լ���Ӧ������HCl��������ǻ�����Ӧ������Ӧѡ��NaHCO3��Һ����ѡb����Ϊ��b��
(3)������A�������������ܣ�������ƿ�м������Ƭ���ɷ�ֹ���С���Ϊ�������ܣ���ֹ���У�
�ڲ���3�У���5%Na2CO3��Һϴ�ӣ���Ҫ��ȥ��Ȳ��л�����Na2CO3��Һ�����ܡ�������л���IJ�������Ϊ��Һ����Ϊ����Ȳ���Һ��
�۲���4�У�����ʱ������ˮԡ���ȵ�ԭ���DZ�Ȳ������ķе��ˮ�ĸߡ���Ϊ����Ȳ������ķе��ˮ�ĸߡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��ش��������⣺
(1) �к��еĹ�����Ϊ__________��
�к��еĹ�����Ϊ__________��
(2) ��ϵͳ����Ϊ___________
��ϵͳ����Ϊ___________
(3)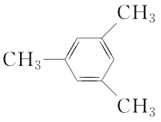 ������Ϊ___________��___________
������Ϊ___________��___________
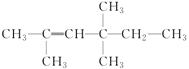
(4)�л���(CH3) 3CCH2CH(C2H5)CH3����Ϊ2,2��������4���һ����飬�����ԭ����___________������ȷ��ϵͳ����ӦΪ___________��
(5)����������CH3CH2CH3��CH3CH=CH2��CH3C��CH��![]() ��(CH3)2CHCH3��
��(CH3)2CHCH3��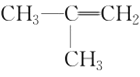 ��
��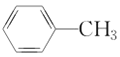 ��
��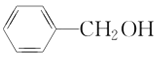 ��
�� ����CH3CH2Cl �����ڱ���������___________�����ڱ���ͬϵ�����___________����Ϊͬϵ�����___________����Ϊͬ���칹�����___________��
����CH3CH2Cl �����ڱ���������___________�����ڱ���ͬϵ�����___________����Ϊͬϵ�����___________����Ϊͬ���칹�����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��������������ԭ�����͵��ǣ� ��
A.��ӦCO(g)+NO2(g)![]() CO2(g)+NO(g)������ӦΪ���ȷ�Ӧ������ƽ��������¶���ϵ��ɫ����
CO2(g)+NO(g)������ӦΪ���ȷ�Ӧ������ƽ��������¶���ϵ��ɫ����
B.����2HI(g) ![]() H2(g) +I2(g)����ƽ�����С���������ʹ��ϵ��ɫ����
H2(g) +I2(g)����ƽ�����С���������ʹ��ϵ��ɫ����
C.��ˮ�м���NaOH���������ڰ��������
D.�ϳɰ���Ӧ��Ϊ��߰��IJ��ʣ�������Ӧ��ȡ�����¶ȵĴ�ʩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڵ��ұ�����Ĺ����м������ʯ(Na3AlF6)������Al2O3�۵�����á�
��1����̬��ԭ���У�����ռ�ݵ�����ܲ�ķ���Ϊ______������ռ������ܼ��ϵĵ�����Ϊ______��
��2����NaAlO2����������Һ��ͨ��CO2�����Ƶñ���ʯ��
�ٸ÷�Ӧ���漰�ķǽ���Ԫ�صĵ縺���ɴ�С��˳��Ϊ______________��
��1molCO2�к��е�������ĿΪ________������Cԭ�ӵ��ӻ���ʽΪ_________��CO2��SCN����Ϊ�ȵ����壬SCN���ĵ���ʽΪ_________��
��Na2O���۵��NaF�ĸߣ��������ǣ�_________��
��3������ʯ�����������ɣ�����ʯ�ľ����ṹ��ͼ��ʾ����λ�ڴ�������Ķ�������ģ���λ�ڴ��������12������е��8��С����������ģ���ô������������Ĵ���������������________(��������)��
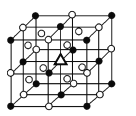
��4������ʯϡ��Һ�д��ڵĻ�ѧ����________(����)��
A ���Ӽ� B ���ۼ� C ��λ�� D ���
��5�������������е�ԭ�Ӷѻ���ʽΪ�����������ܶѻ����侧������Ϊapm������ԭ�ӵİ뾶Ϊ______pm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦN2(g)��O2(g)��2NO(g)�������仯��ͼ��ʾ��
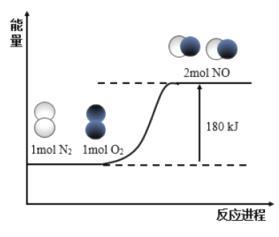
��֪���Ͽ�1mol N2(g)�л�ѧ��������946kJ�������Ͽ�1mol O2(g)�л�ѧ��������498kJ����������˵����ȷ����
A.�Ͽ�1mol NO(g)�л�ѧ����Ҫ����632kJ����
B.NO(g)��![]() N2(g)��
N2(g)��![]() O2(g) H����90kJ/mol
O2(g) H����90kJ/mol
C.N2(g)��O2(g)��2NO(g) H����180kJ/mol
D.�γ�1mol NO(g)�л�ѧ��ʱ�ͷ�90kJ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧģ�ҵ�Ƶ�ķ�����̽��ClO3����I���ķ�Ӧ���ɡ�ʵ��������������£�
ʵ�鼰�Լ� | ��� | ��ɫNaClO3��Һ���� | �Թ�����Һ��ɫ | ����KI��ֽ��ɫ |
| 1 | 0.05mL | dz��ɫ | ��ɫ |
2 | 0.20mL | ���ɫ | ��ɫ | |
3 | 0.25mL | dz��ɫ | ��ɫ | |
4 | 0.30mL | ��ɫ | ��ɫ |
(1)ȡʵ��2�����Һ����������ʵ�飺
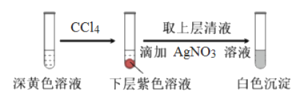
�����飬������ɫ������AgCl��д������0.20mLNaClO3����Һ��ClO3����I��������Ӧ�����ӷ���ʽ____________��
(2)�������ϣ�һ�������£�I����I2�����Ա�������IO3����
�������裺NaClO3��Һ�������ӵ�����Һ��ɫ��ԭ���ǹ�����NaClO3��Һ��(1)�еķ�Ӧ���������Ӧ��ͬʱ����Cl2����Ӧ�����ӷ���ʽ��______________________��
����ʵ�飺ȡ����ʵ��4�е���ɫ��Һ��������ʵ�飬��һ����֤���к���IO3���������Լ�X������_________(����ĸ���)��
a����ˮ b��KMnO4��Һ c��NaHSO3��Һ
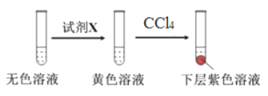
��ý��ۣ�NaClO3��Һ�������ӵ�����Һ��ɫ��
(3)С��ͬѧ����ʵ�飬ͨ���ı�ʵ��4��������Һ���������������ʵ������
��� | 6.0mol��L��1H2SO4��Һ���� | �Թ�����Һ��ɫ | ����KI��ֽ��ɫ |
5 | 0.25mL | dz��ɫ | ��ɫ |
6 | 0.85mL | ��ɫ | ��ɫ |
�ٶԱ�ʵ��4��5�����Ի�õĽ�����______________________��
��ʵ��6��ClO3����I����Ӧ�����ӷ���ʽ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������(Na2S2O4)�׳Ʊ��շۣ���һ�ֵ���ɫ��ĩ��������ˮ���������Ҵ�����ʵ�����Ʊ��������������������£�
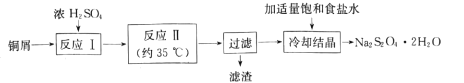
(1)��Ӧ�����Ʊ�SO2����ͼװ�ÿ���ȡ���������SO2��
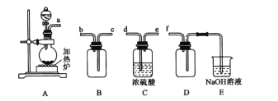
�ٰ������������Ӹ������ӿڣ�˳��Ϊa�� ___��f��װ��D��������______��
��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ___��
(2)��Ӧ������ʵ��װ����ͼ��ʾ(����װ��ʡ��)��
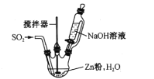
��ͨSO2֮ǰ��ǿ�����裬��п�ۺ�ˮ�Ƴ�����Һ����Ŀ����_________�����Ʒ�Ӧ�¶ȵķ�����____
�ڷ�Ӧ������ӷ���ʽΪ ___��
(3)����������ϴ�ӡ����գ��ɵõ�һ�ֹ�ҵ��Ʒ��____(�ѧʽ)��������������ʳ��ˮ��Ŀ���� ___��
(4)��ƷNa2S2O42H2O���ÿ������ױ����������������������_______(д2��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й����ʵı���ʽ��ȷ����
A. ��Ȳ���ӵı���ģ��ʾ��ͼ��![]()
B. 2������2����ϩ�ļ���ʽ��![]()
C. ![]() �����ƣ�3-��-1-��ϩ
�����ƣ�3-��-1-��ϩ
D. 1��3������ϩ�ķ���ʽ��C4H8
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��3H2(g)+N2(g)2NH3(g)��H=-92kJ/mol���ڴ�������ʱ��Ӧ�����е������仯��ͼ��ʾ������������ȷ����
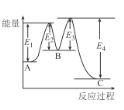
A.��H=E2-E1+E3-E4
B.���������Ӧ����������ɣ����е�һ����Ӧ�����ܷ�Ӧ����
C.�����������H����Ӧ���ʾ������ı�
D.���ܱ������г���3 mol H2��1molN2������������Ӧ���ﵽƽ��ʱ����Ӧ�ų�92 kJ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com