
�±�Ϊ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�
| A | |||||||||||||||||
| B | C | D | E | ||||||||||||||
| F | G | H | |||||||||||||||
| I | J |
��ش����и��⣺
��1����A��B��C�γɵ�ABC�����У����ЦҼ���м�������Ϊ ��
��2��BD2��GD2�ĸ���������ˮ�� ��
ԭ��
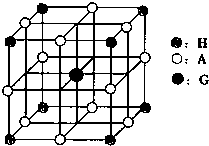
��3����֪Ԫ��E��I�γɾ�����ͼ2��ʾ����I2+����λ���� �� E������λ���� ��


|
��4��JCl3����C��D���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����硣��JCl3�γɵ������Ļ�ѧʽΪ
��5����֪����FH�ľ����ṹ��ͼ1��ʾ���þ�����ܶ�Ϊ![]() �������ӵ�����ΪNA����þ��������ڵ�����F+��H���ĺ˼��Ϊ
�������ӵ�����ΪNA����þ��������ڵ�����F+��H���ĺ˼��Ϊ
���г���ʽ���ɣ�
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2011?������ģ��X��Y��Z��W��Ϊ����������Ԫ�أ�X��W��������֮��Ϊ23���±�Ϊ���ڱ���һ���֣�����˵����ȷ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
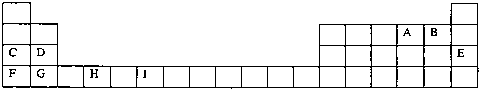
| m | n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ��ˮ��ѧ�߶�5��ѧ���϶�ģ���⻯ѧ�Ծ����������� ���ͣ������
�±�Ϊ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء���ش��������⣺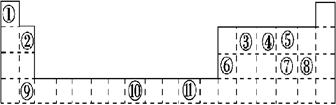
(1)��������ds����Ԫ����_____ (����)��
(2)  ��۵����Ų�ʽ__________�����������е����Ų�4s����ϵ�������֮��ͬ�Ļ��� Ԫ��(��Ԫ�ط���)��
��۵����Ų�ʽ__________�����������е����Ų�4s����ϵ�������֮��ͬ�Ļ��� Ԫ��(��Ԫ�ط���)��
(3)ijԪ�ص����������Ų�ʽΪnsnnpn��1����Ԫ��ԭ�ӵĺ����������ӳɶԵ���Ϊ_ _�ԡ�
(4)�Ƚ�������ֵ�Ĵ�С(ѡ�>����<����=��)
��һ�����ܣ��� �ݣ��縺�ԣ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��13�֣��±�Ϊ���ڱ���һ���֣��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
|
a |
|
|
|
|
|||||||||||||
|
|
|
|
|
b |
c |
d |
|
|
|||||||||
|
|
|
|
|
|
e |
f |
|
|
|||||||||
|
|
|
|
|
|
g |
|
h |
|
|
|
|
|
|
|
|
|
|
�û�ѧ����ش��������⣺
��1��д��Ԫ��g�Ļ�̬ԭ�Ӻ�������Ų�ʽ___________________________��
h2+��δ�ɶԵ�����Ϊ ��
��2����b2a2�����У�Ԫ��bΪ �ӻ����÷����� ���ӣ�����ԡ��Ǽ��ԡ������÷����ЦҼ��ͦм�����Ŀ��Ϊ ��
��3�� bd2��bf2�Ƚϣ��е�ϸߵ���_______�������ʽ����ԭ���� ��
��4�����й���Ԫ����Ԫ�����ڱ��е�λ���Լ�Ԫ��ԭ�ӵ���Χ�����Ų��ص���й�����ȷ�� ��
A��hλ��Ԫ�����ڱ��е������ڵ�VIII�壬����d��Ԫ��
B��e�Ļ�̬ԭ���У�3p�ܼ�Ϊ�����������p��Ԫ��
C�����������Ų�ʽΪ4s2��һ������IIA��
D�����������Ų�ʽΪns2np1����Ԫ�ؿ����Ǣ�A����B��
��5����ѧ�о�������Ԫ��b��Ԫ��c�����γ�һ�ֳ�Ӳ����ĥ�����µ��������ǽ������ϣ��仯ѧʽΪ �����۵�Ƚ��ʯ ����ߡ��͡�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com