
| n(OH-)-n(H+) |
| V(��)+V(��) |
| 0.12-0.1 |
| 2 |
| n(OH-)-n(H+) |
| V(��)+V(��) |
| 10-2mol/L��(b-a)L |
| (a+b)L |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1�������£���0.1 mol��L-1�Ĵ�����Һ��0.1 mol��L-1��������Һ�������ϣ������Һ�����ԣ���û��Һ��c��CH3COOH��_____________c��CH3COO-����c��Na+��+c��H+��___________c��CH3COO-��+c��OH-��
��2�������£���0.1 mol��L-1��HCN��Һ��0.1 mol��L-1��NaCN��Һ�������ϣ���û��Һ��c��HCN����c��CN-�����������Ϲ�������Һ����ĸı���Բ��ƣ�����û��Һ��pH________7��c��HCN��+c��CN-��0.1 mol��L-1��![]() __________c��OH-����
__________c��OH-����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������м���һ�и߶��������¿���ѧ�Ծ����������� ���ͣ������
��ѡ���⣩��10�֣��ں���������ʵ���Һ�У������ж����ѧƽ��档
��1�������£���0.2mol/L��ijһԪ��HA��Һ��0.1mol/L NaOH��Һ�������Ϻ���ҺpH����7�������Һ�����������Һ���֮�ͣ�����Һ�����й�ϵ��ȷ����____
A��c(HA)��c(A��) B��c(HA)һ������0.1mol/L
C��c(Na+)��c(HA)+c(A��) D��2c��OH������2c(H+)��[c(HA)��c(A��)]
��2����������20mL0.1mol/L Na2CO3��Һ����μ���0.1mol/L HCl��Һ40mL����Һ�к�̼Ԫ�صĸ�������CO2�ݳ�δ�������ʵ������������ᣩ����ҺpH�仯�IJ��������ͼ��ʾ��
�ش��������⣺
����ͬһ��Һ�У�H2CO3��HCO3����CO32��������ܡ����ܡ��� �������档
�ڵ�pH=7ʱ����Һ�и������������ʵ���Ũ�ȵĴ�С��ϵ�ǣ�
��
����֪��25��ʱ��CO32��ˮ�ⷴӦ��ƽ�ⳣ����ˮ�ⳣ��Kh= 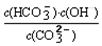 =2��10��4������Һ��c(HCO3��)�Uc(CO32��)=2�U1ʱ����Һ��pH= ��
=2��10��4������Һ��c(HCO3��)�Uc(CO32��)=2�U1ʱ����Һ��pH= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������и߶��������¿���ѧ�Ծ��������棩 ���ͣ������
��ѡ���⣩��10�֣��ں���������ʵ���Һ�У������ж����ѧƽ��档
��1�������£���0.2mol/L��ijһԪ��HA��Һ��0.1mol/L NaOH��Һ�������Ϻ���ҺpH����7�������Һ�����������Һ���֮�ͣ�����Һ�����й�ϵ��ȷ����____
A��c(HA)��c(A��) B��c(HA)һ������0.1mol/L
C��c(Na+)��c(HA)+c(A��) D��2c��OH������2c(H+)��[c(HA)��c(A��)]
��2����������20mL0.1mol/L Na2CO3��Һ����μ���0.1mol/L HCl��Һ40mL����Һ�к�̼Ԫ�صĸ�������CO2�ݳ�δ�������ʵ������������ᣩ����ҺpH�仯�IJ��������ͼ��ʾ��
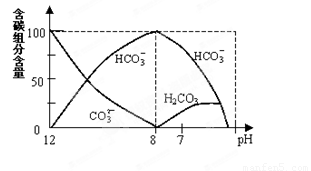
�ش��������⣺
����ͬһ��Һ�У�H2CO3��HCO3����CO32��������ܡ����ܡ��� �������档
�ڵ�pH=7ʱ����Һ�и������������ʵ���Ũ�ȵĴ�С��ϵ�ǣ�
��
����֪��25��ʱ��CO32��ˮ�ⷴӦ��ƽ�ⳣ����ˮ�ⳣ��Kh= 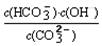 =2��10��4������Һ��c(HCO3��)�Uc(CO32��)=2�U1ʱ����Һ��pH=
��
=2��10��4������Һ��c(HCO3��)�Uc(CO32��)=2�U1ʱ����Һ��pH=
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com