
��1��2SO2(g)+O2(g)  2SO3(g) ��Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2(g)����Ϊ1mol SO3(g)�Ħ�H= �C99kJ��mol����ش��������⣺
2SO3(g) ��Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2(g)����Ϊ1mol SO3(g)�Ħ�H= �C99kJ��mol����ش��������⣺
��ͼ��A���ʾ �� C���ʾ ��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ�� ������С����ޡ���Ӱ�졣
��ͼ�С�H= kJ/mol��
��2��25�桢101 kPa�£�2g����ȼ������Һ̬ˮ���ų�285.8kJ��������������ȼ���ȵĻ�ѧ����ʽΪ ��
��3����C(s) + O2(g) �� CO2(g)����H = �C393.5kJ/mol
��CO(g) + 1/2 O2(g) �� CO2(g)����H = �C283kJ/mol
����������Ϣ��д��Cת��ΪCO���Ȼ�ѧ����ʽΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | CO | H2 | CH3OH |
| Ũ�ȣ�mol?L-1�� | 0.9 | 1.0 | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


| YO2 | YO3 | |
| ���� | 94%��95% | 5%��6% |
| ���� | 97%��98% | 2%��3% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 | 2 |
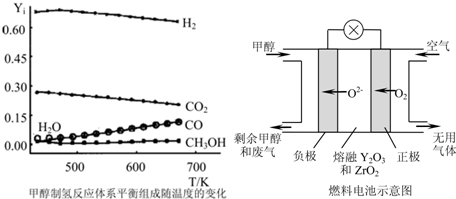
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡ����10���¿���ѧ�Ծ��������棩 ���ͣ������
(14��) �о�NO2��SO2 ��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
(1)���÷�Ӧ6NO2��8NH3  7N2��12H2O�ɴ���NO2����ת��3.6mol����ʱ�����ɵ�N2�ڱ�״������
L��
7N2��12H2O�ɴ���NO2����ת��3.6mol����ʱ�����ɵ�N2�ڱ�״������
L��
(2)��֪����Ӧ1��2SO2(g)+O2(g) 2SO3(g) ��H = ��196.6 kJ��mol-1
2SO3(g) ��H = ��196.6 kJ��mol-1
��Ӧ2��NO2(g)+SO2(g) SO3(g)+NO(g) ��H = ��41.8kJ��mol-1
SO3(g)+NO(g) ��H = ��41.8kJ��mol-1
��3��2NO(g)+O2(g) 2NO2(g)�� ��H
= _________ kJ��mol-1
2NO2(g)�� ��H
= _________ kJ��mol-1
(3) һ�������£���2molNO��2molO2���ں����ܱ������з���������Ӧ3�����и�����˵����Ӧ�ﵽƽ��״̬���� ��
a����ϵѹǿ���ֲ��� b�����������ɫ���ֲ���
c��NO��O2�����ʵ���֮�ȱ��ֲ��� d��ÿ����1 molO2ͬʱ����2 molNO2
(4)CO�����ںϳɼ״���һ���¶��£������Ϊ2L���ܱ������м���CO��H2��������ӦCO��g��+2H2��g�� CH3OH��g������ƽ����ø����Ũ�����£�
CH3OH��g������ƽ����ø����Ũ�����£�
|
���� |
CO |
H2 |
CH3OH |
|
Ũ��(mol•L��1) |
0.9 |
1.0 |
0.6 |
�ٻ�������ƽ����Է�������__________________________��
����ʽ������ƽ�ⳣ��K=__________________________��
�������������ѹ��Ϊ1L,�������㣬Ԥ����ƽ����c(H2)��ȡֵ��Χ��__________��
��������������䣬�ٳ���0.6molCO��0.4molCH3OH,��ʱv��___v��(�>������<����=��)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com