
 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���¸�ѹ |
| ���� |
| ���¸�ѹ |
| ���� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���½���³ľ�������������������Բ��ԣ����ۣ���ѧ���� ���ͣ��ۺ���
ũ��������������Ҫ���⡢������ˮ������Ҫ���ֻ�ѧԪ�ء���ֲ��ȱ��NԪ��ʱ������Ϊֲ������������Ҷɫ���ƣ�����ʱҶƬ����ֱ��������
��1�����п������ʵĻ������� �����к�N����ߵ��� ��
A������� B���������� C������� D������
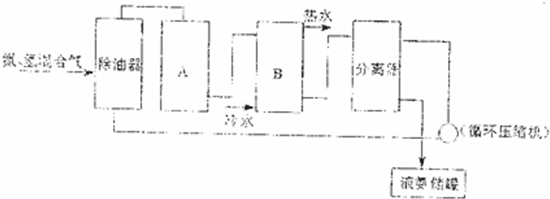
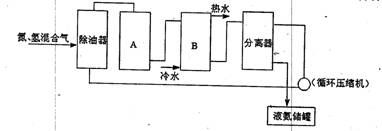
��2�����ʵ��Ʊ��������漰���ĺϳɣ�����д���кϳɰ���������ͼ�е��豸���ƣ�
A B
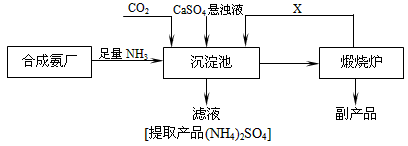
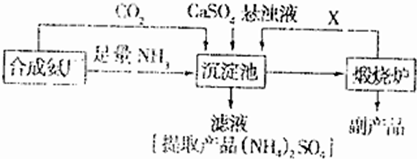
��3��ij������Ϊ���ۺ��������������еĸ���ƷCaSO4�������ڵĺϳɰ�����������������Ʊ���NH4��2SO4�Ĺ������̣�
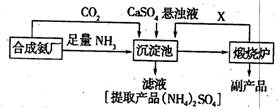
�ٳ������з�������Ҫ��Ӧ����ʽ�� ��
�����������̵ij�������ͨ������������Ŀ���� ������ѭ��ʹ�õ�X�� ��
�۴����ʵ����ʺ�ҵ����ʵ�ʵĽǶȿ��Ǹ����̵���Ҫȱ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com