
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
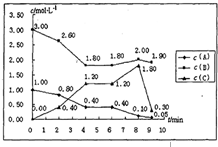 ��һ���ݻ��̶�Ϊ1L���ܱ������У�������ӦmA��g��+nB��g���TpC��g����H=������Ӧ�������ͼ��ʾ��
��һ���ݻ��̶�Ϊ1L���ܱ������У�������ӦmA��g��+nB��g���TpC��g����H=������Ӧ�������ͼ��ʾ��| 10 |
| 9 |
| 10 |
| 9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| 32V |
| 22.4W |
| 22.4V |
| 32W |
| 3(W-m) |
| W |
| 8(W-m) |
| 5W |
| ���� |
| �� |
| ���� |
| �� |
| 1 |
| 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
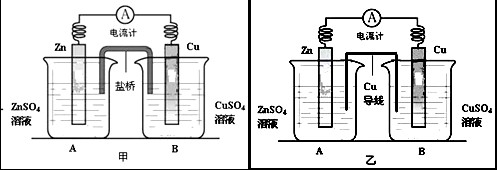
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����Ʊ���һ��ѧ³�ư� ³�ư� ���ͣ�022
Ħ������������Ħ�����
1��Ħ������
��λ���ʵ��������������е���������Ħ������(����M)��ȷ����Ħ�������ĸ����ע�����¼��㣺
(1)��λ��SI�У�Ħ�������ĵ�λ��kg��mol��1����ѧ�γ���________�����⣬����kg��kmol��1��mg��mmol��1�ȣ����У�1 g��mol��1��1 kg��kmol��1��1 mg��mmol��1��
(2)���ţ�Ħ�������ķ�����M����������Ħ�������ɱ�ʾΪ________��
(3)��ֵ����Ħ��������g��mol��1Ϊ��λʱ��
M��Mr g��mol��1��M��Ar g��mol��1��
�����ǵĴ���ֵ����ȵģ�
(4)��Ħ�������ĸ���õ����й�ʽ��n��![]()
2������Ħ�����
��һ�����¶Ⱥ�ѹǿ�£���λ���ʵ���������ռ�������������Ħ�����(����Vm)��
��״���£������Ħ�����(Vm��0)ԼΪ22.4 L��mol��1��
ע�⣺(1)����Ħ��������ǡ����������λ���������λL��m3�ȣ��䳣�õ�λΪ��L��mol��1����SI��λΪm3��mol��1��
(2)������Ħ������ĸ���ɳ�������Ħ������Ĺ�ʽ����Vm��![]() (��n��
(��n��![]() )
)
(3)�����С���λ���ʵ�����������1 mol��Ҳ������1 mmol��1 kmol�ȣ�
3��Ӱ���������������
(1)���������������Ķ��٣���������������________��________Խ��
(2)�����������ӵ�________��
(3)���������Ӽ��________��
�Թ����Һ��������˵��(1)(2)����Ҫ���ã�������������˵��(1)(3)����Ҫ���ã�
4�������ӵ�����
����ͬ���¶Ⱥ�ѹǿ�£�________��������ɽ��������ӵ����ɣ�
ȷ���Ⱒ���ӵ�����Ҫע�����¼��㣺
(1)ʹ�÷�Χ�������ӵ����ɵ�ʹ�÷�Χ��________���ʣ������ǵ�һ���壬Ҳ���ǻ�����壮
(2)������ʽ�������ӵ����ɵ������ǡ���ͬ������ͬ�¡�ͬѹ��ͬ�����ͬ��������������������ͬ�����ĸ�����Ȼ��ͬ���������ӵ����ɿ��������ֱ�����ʽ��
�ٵ�T��p��N(��n)��ͬʱ��V����ͬ��
�ڵ�T��p��V��ͬʱ��N(��n)����ͬ��
�۵�T��V��N(��n)��ͬʱ��p����ͬ��
�ܵ�p��N(��n)��V��ͬʱ��T����ͬ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com