
��14�֣���ҵ������CO������������֪��25��ʱ��
C(s,ʯī) + 1/2 O2(g) = CO(g) ��H1= -111kJ/mol
H2(g) + 1/2 O2(g) = H2O(g) ��H2= -242kJ/mol
C(s,ʯī) + O2(g) = CO2(g) ��H3= -394kJ/mol
��1����25��ʱ��CO(g)
+ H2O(g)  CO2(g) + H2(g) ��H=_____________��
CO2(g) + H2(g) ��H=_____________��
��2����2L�ܱ������У���2
mol CO��3 mol H2O��ϼ��ȵ�800�棬�������з�Ӧ��CO(g)+H2O(g)  CO2(g)+H2(g) K=1.0����ƽ���CO��ת����Ϊ_______��ƽ��������H2���������Ϊ_______��
CO2(g)+H2(g) K=1.0����ƽ���CO��ת����Ϊ_______��ƽ��������H2���������Ϊ_______��
��3������2���е�ƽ���Ļ������ͨ��300mL 6mol/L NaOH��Һ�У�������գ�������Һ������Ũ���ɴ�С��˳��Ϊ_________________________________________________��
��4������3����ʣ�������ͨ������Ũ������������������õ����ȼ��ͨ�������Ĺ��������У��������Ƶ���������______g��
��5����ҵ��Ҳ������CO��H2�����״���CO(g) + 2H2 (g) = CH3OH (g)����һ�������£��÷�Ӧ��һ���ܱ������дﵽƽ�⣬��ά��c(H2)���������¶Ȳ��䣬�����������������ƽ��_________������ĸ��
A�����ƶ� B��������Ӧ�����ƶ�
C�����淴Ӧ�����ƶ� D�����ж��ƶ��ķ���
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�긣��ʡȪ���и����ʼ����ۻ�ѧ�Ծ��������棩 ���ͣ������
̼���仯����Ӧ�ù㷺��
I.��ҵ������CO��ˮ������Ӧ����������������ƽ��:CO(g)+H2O(g)  CO2(g)+H2(g)
CO2(g)+H2(g)
��1����ʯ����ɸ�к��й�Ԫ�أ���д����ԭ�ӽṹʾ��ͼ__________��
��2����1L�����ܱ�������ע��CO��H2o(g),830��ʱ��ò����������±�������¶��·�Ӧ��ƽ�ⳣ
��K=______________��

��3����ͬ�����£���1L�����ܱ������У�ͬʱע��1mol CO��1mol H2O(g),2molCO2��2mo1 H2����ʱv(�� ) __________v(��)(���������������)
II.��֪CO(g)+1/2 O2 (g)��CO2 (g)????????????? ��H��һ141 kJ��mol-1
2H2(g)+ O2(g)��2H2O(g)??????????????????????? ��H��һ484 kJ��mol-1
CH3OH��1��+3/2O2 (g)��CO2(g)+2H2O(g)????????? ��Hl��һ726 kJ��mol-1
��4������CO��H2�����Ƶ�Һ̬�״����Ȼ�ѧ����ʽΪ___________��
III.һ����������ȼ�ϵ�ع���ԭ������ͼ��ʾ
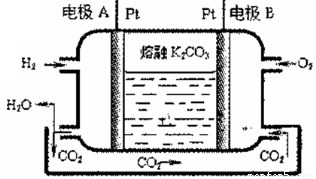
��5��д���缫A�ĵ缫��Ӧʽ_____________��
��6����������ص�ⱥ��ʳ��ˮ��������0.2mo1 Cl2����������ͨ��O2�����Ϊ_____L(��״��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ҫ�ĵ��ʣ��Dz������Ļ�����Ʒ֮һ����ϳ�ԭ��Ϊ
N2��g��+3H2��g��![]() 2NH3��g�� ��H=-92��4KJ/moL���Իش��������⣺
2NH3��g�� ��H=-92��4KJ/moL���Իش��������⣺
���ĺϳ���һ�����淴Ӧ��Ϊ��̽���ϳɰ���������������ǽ����˴������о���
��1�� �����й����������˵���( )
A��������ѭ������ʹδת���ĵ����������õ���ֵ����á�
B ���ۺϿ��Ǹ���������أ�ѹǿ������20�D50MPa֮��Ϊ��
C�����õ��ˮ����������Ϊ�ϳɰ���ԭ�ϣ�
D��ʹ������ýΪ��������5000Cʱ�������Ч�档
��2���ϳɰ���ҵ�в�ȡ�����д�ʩ������������ԭ�����͵��� ������ţ�
A �����ýϸ�ѹǿ��20�D50MPa��
B������5000C�ĸ���
C��������ýΪ����
D���������İ�Һ������ʱ����ϵ�з��������N2��H2ѭ�����ϳ����в�����N2��H2
��3����298Kʱ����10moL N2��30moL H2����ϳ����У�Ϊ�ηų�������С��924KJ��
��4������֪���ںϳɰ���ҵ�а������ɣ�
�����ߵ�λʱ���ڰ����IJ��������¶ȶ��ԣ��� ������»���£���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣���ҵ������CO������������֪��25��ʱ��
C(s,ʯī) + 1/2 O2(g) = CO(g) ��H1=-111kJ/mol
H2(g) + 1/2 O2(g)= H2O(g) ��H2= -242kJ/mol
C(s,ʯī) + O2(g) = CO2(g) ��H3=-394kJ/mol
��1����25��ʱ��CO(g)+ H2O(g) CO2(g) + H2(g) ��H=_____________��
��2����2L�ܱ������У���2mol CO��3 mol H2O��ϼ��ȵ�800�棬�������з�Ӧ��CO(g)+H2O(g) CO2(g)+H2(g) K=1.0����ƽ���CO��ת����Ϊ_______��ƽ��������H2���������Ϊ_______��
��3������2���е�ƽ���Ļ������ͨ��300mL 6mol/L NaOH��Һ�У�������գ�������Һ������Ũ���ɴ�С��˳��Ϊ_________________________________________________��
��4������3����ʣ�������ͨ������Ũ������������������õ����ȼ��ͨ�������Ĺ��������У��������Ƶ���������______g��
��5����ҵ��Ҳ������CO��H2�����״���CO(g) + 2H2 (g) = CH3OH(g)����һ�������£��÷�Ӧ��һ���ܱ������дﵽƽ�⣬��ά��c(H2)���������¶Ȳ��䣬�����������������ƽ��_________������ĸ��
A�����ƶ� B��������Ӧ�����ƶ�
C�����淴Ӧ�����ƶ� D�����ж��ƶ��ķ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�������ʡ�����и���������ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��14�֣���ҵ������CO������������֪��25��ʱ��
C(s,ʯī) + 1/2 O2(g) =" CO(g) " ��H1= -111kJ/mol
H2(g) + 1/2 O2(g) = H2O(g) ��H2= -242kJ/mol
C(s,ʯī) + O2(g) = CO2(g) ��H3= -394kJ/mol
��1����25��ʱ��CO(g) + H2O(g)  CO2(g) + H2(g) ��H=_____________��
CO2(g) + H2(g) ��H=_____________��
��2����2L�ܱ������У���2 mol CO��3 mol H2O��ϼ��ȵ�800�棬�������з�Ӧ��CO(g)+H2O(g) CO2(g)+H2(g) K=1.0����ƽ���CO��ת����Ϊ_______��ƽ��������H2���������Ϊ_______��
CO2(g)+H2(g) K=1.0����ƽ���CO��ת����Ϊ_______��ƽ��������H2���������Ϊ_______��
��3������2���е�ƽ���Ļ������ͨ��300mL 6mol/L NaOH��Һ�У�������գ�������Һ������Ũ���ɴ�С��˳��Ϊ____________________________________ _____________��
_____________��
��4������3����ʣ�������ͨ������Ũ������������������õ����ȼ��ͨ�������Ĺ��������У��������Ƶ���������______g��
��5����ҵ��Ҳ������CO��H2�����״���CO(g) + 2H2 (g) = CH3OH (g)����һ�������£��÷�Ӧ��һ���ܱ������дﵽƽ�⣬��ά��c(H2)���������¶Ȳ��䣬
(g) = CH3OH (g)����һ�������£��÷�Ӧ��һ���ܱ������дﵽƽ�⣬��ά��c(H2)���������¶Ȳ��䣬 �����������������ƽ��_________������ĸ��
�����������������ƽ��_________������ĸ��
| A�����ƶ� | B��������Ӧ�����ƶ� |
| C�����淴Ӧ�����ƶ� | D�����ж��ƶ��ķ��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com