
���ӹ�ҵ����30����FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�壮��ش��������⣮
(1)������Һ��Fe3+���ڵ��Լ��ǣ�________������________________��
(2)д��FeCl3��Һ�����ͭ������Ӧ�����ӷ���ʽ��________��
����ݸ÷�Ӧ���һ��ԭ��أ��ڷ����л�������װ��ͼ��(����缫���ơ��缫���ϡ��������Һ)

(3)ij����ʦΪ�˴�ʹ�ù��ĸ�ʴ��Һ�л���ͭ�������»�ô�����FeCl3��Һ�����������в��裺

A����д������ʵ�����й����ʵĻ�ѧʽ��
��________����________����________
B����д������ʵ������ط�Ӧ�����ӷ���ʽ��
________________________
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
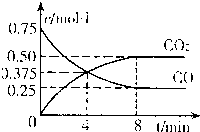 ��2010?Ϋ��һģ����Ԫ�ؼ��仯�������������������ϢϢ��أ�
��2010?Ϋ��һģ����Ԫ�ؼ��仯�������������������ϢϢ��أ�| 1 | 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013��㶫½����ʯ��ѧ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ����������� ���ͣ�ʵ����
(I) ���ӹ�ҵ����30����FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�塣��FeCl3��Һ����ʴҺ��Cu��Ӧ����CuCl2��FeCl2��
(1) д���÷�Ӧ�Ļ�ѧ����ʽ ��
(2) ������Һ��Fe3+���ڵ��Լ��� ��֤��Fe3+���ڵ������� ��
(��) ӡˢ��·�ķϸ�ʴҺ���д���CuCl2��FeCl2��FeCl3�������ŷŻ���ɻ�����Ⱦ����Դ���˷ѡ�ͨ���������̿ɴӸ÷�Һ�л���ͭ���������Ļ�����ȫ��ת��ΪFeCl3��Һ����Ϊ��ʴҺԭ��ѭ��ʹ�á�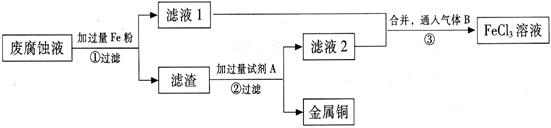
�� ������з�����Ӧ�����ӷ���ʽ ��
(2) ������������Լ�A�� (�ѧʽ)��
(3) �����ͨ�������B�� (�ѧʽ)��д���÷�Ӧ�Ļ�ѧ����ʽ ��
(4) Ϊ�ⶨ������ͭ������������ȡ50g���������������Լ�A��Ӧ���õ�5.6L���壨��״���£����Լ���������ͭ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ������������������һ��ѧ��ѧҵ���г�����ۻ�ѧ�Ծ��������棩 ���ͣ������
��Ԫ�ؼ��仯�������������������ϢϢ��أ��Իش��������⣺
��1�����ӹ�ҵ����30����FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�壬�÷�Ӧ�����ӷ���ʽΪ ��
��2����֪��Fe(s)+ O2(g)
O2(g) FeO(s)
��H=��272 kJ��mol��1
FeO(s)
��H=��272 kJ��mol��1
C(s)+O2(g) CO2(g) ��H=��393.5 kJ��mol��1
CO2(g) ��H=��393.5 kJ��mol��1
2C(s)+O2(g) 2CO(g) ��H=��221 kJ��mol��1
2CO(g) ��H=��221 kJ��mol��1
���¯����������
FeO(s)+CO(g) Fe(s)+CO2(g) ��H=
��
Fe(s)+CO2(g) ��H=
��
��3�����죨Fe2O3����һ�ֺ�ɫ���ϡ���һ��������������160mL 5 mol��L��1�����У��ټ����������ۣ�����Ӧ�������ռ�������2.24L����״�������������Һ����Fe3������μӷ�Ӧ�����۵�����Ϊ ��
��4����H2��O2��������Na2CO3���ȼ�ϵ�أ����õ�ⷨ�Ʊ�Fe(OH)2��װ������ͼ��ʾ������P��ͨ��CO2��
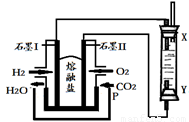
��ʯīI�缫�ϵĵ缫��ӦʽΪ ��
��ͨ��һ��ʱ����Ҳಣ�����в��������İ�ɫ�������ҽϳ�ʱ�䲻��ɫ��������˵������ȷ���� ������ţ���
A��X��Y���˶������������缫
B��������NaOH��Һ��Ϊ���Һ
C�����������ķ�Ӧ�ǣ�2H2O�� 2e��= H2��+ 2OH��
D����ɫ����ֻ���������ϲ���
����������Fe(OH)2������¶�ڿ����У�����ɫ�仯Ϊ ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��㶫½����ʯ��ѧ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
(I) ���ӹ�ҵ����30����FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�塣��FeCl3��Һ����ʴҺ��Cu��Ӧ����CuCl2��FeCl2��
(1) д���÷�Ӧ�Ļ�ѧ����ʽ ��
(2) ������Һ��Fe3+���ڵ��Լ��� ��֤��Fe3+���ڵ������� ��
(��) ӡˢ��·�ķϸ�ʴҺ���д���CuCl2��FeCl2��FeCl3�������ŷŻ���ɻ�����Ⱦ����Դ���˷ѡ�ͨ���������̿ɴӸ÷�Һ�л���ͭ���������Ļ�����ȫ��ת��ΪFeCl3��Һ����Ϊ��ʴҺԭ��ѭ��ʹ�á�

�� ������з�����Ӧ�����ӷ���ʽ ��
(2) ������������Լ�A�� (�ѧʽ)��
(3) �����ͨ�������B�� (�ѧʽ)��д���÷�Ӧ�Ļ�ѧ����ʽ ��
(4) Ϊ�ⶨ������ͭ������������ȡ50g���������������Լ�A��Ӧ���õ�5.6L���壨��״���£����Լ���������ͭ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����Ͼ����һ��ѧ��һ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(I)���ӹ�ҵ����30����FeCl3����Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�塣��FeCl3��Һ����ʴҺ��Cu��Ӧ����CuCl2��FeCl2��
(1)д���÷�Ӧ�Ļ�ѧ����ʽ ��
(2)������Һ��Fe3+���ڵ��Լ���
(��)ӡˢ��·�ķϸ�ʴҺ���д���CuCl2��FeCl2��FeCl3�������ŷŻ���ɻ�����Ⱦ����Դ���˷ѡ�ͨ���������̿ɴӸ÷�Һ�л���ͭ���������Ļ�����ȫ��ת��ΪFeCl3��Һ����Ϊ��ʴҺԭ��ѭ��ʹ�á�
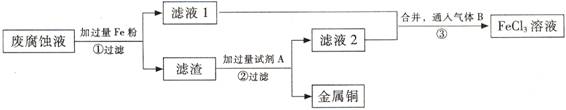 (3) ������з�����Ӧ�����ӷ���ʽ
(3) ������з�����Ӧ�����ӷ���ʽ
(4)��������Ҫ�ɷ��� �� (�ѧʽ)��
(5)Ϊ�˳�ȥ�����е����ʵõ�ͭ���������Լ�A�� (�ѧʽ)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com