�⣺��1�����ӽṹ��H
2O
2���ƣ�S
2Cl
2��չ����ҳ�ͽṹ��Cl-Sλ��������ҳ���ڣ�S
2Cl
2������Sԭ��֮���γ�1�Թ��õ��Ӷԣ�Clԭ����Sԭ��֮���γ�1�Թ��õ��Ӷԣ�����ʽΪ
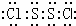
��
���ӽṹ��H
2O
2���ƣ�������ΪҺ�壬���ڷ��Ӿ��壻
�ʴ�Ϊ��
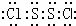
�����Ӿ��壻
��2��S
2Cl
2��ˮ��ˮ�⣬��������ʹƷ����Һ��ɫ�����壬������Ϊ���������ڷ�Ӧ��������Ԫ��һ�������ߵ�+4�ۣ�����SO
2����һ���ֽ��͵�0�ۣ�����S����ͬʱ����HCl����Ӧ����ʽΪ��2S
2Cl
2+2H
2O=3S��+SO
2��+4HCl��
�ʴ�Ϊ��2S
2Cl
2+2H
2O=3S��+SO
2��+4HCl��
��1�����ɹ������̿�֪�����������������������������������ɣ�Ӧ�Ǹ���������������������������������������̣���������������������ɶ������̣����ݿ��Ƶ�PHֵ��ԭ���غ㡢����غ��֪������ˮ�μӷ�Ӧ��ͬʱ�����������ӣ���Ӧ�����ӷ���ʽΪ��MnO
4-+3Fe
2++7H
2O=3Fe��OH��
3��+MnO
2��+5H
+��2MnO
4-+3Mn
2++2H
2O=5MnO
2��+4H
+��
�ʴ�Ϊ��MnO
4-+3Fe
2++7H
2O=3Fe��OH��
3��+MnO
2��+5H
+��2MnO
4-+3Mn
2++2H
2O=5MnO
2��+4H
+��
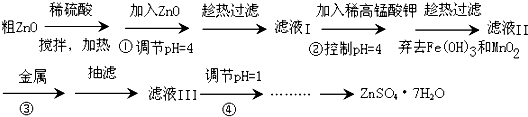
�����̢��м��������Ŀ�ģ�Ӧ�dz�ȥ��Һ�н������ӣ��ֲ����������ʣ�Ӧ��ѡ��п��
�ʴ�Ϊ��C��
��п����ˮ�⣬���̢��е�pH=1Ŀ��������Zn
2+��ˮ�⣬�ʴ�Ϊ������Zn
2+��ˮ�⣻
��2�������չ��壬Ӧ���������ʴ�Ϊ��������
�ڷ�Ӧ����ZnCO
3?2Zn��OH��
2?2H
2O�����ɵ�����Ϊ������̼������Ԫ���غ��֪���������������ɣ���Ӧ����ʽΪ��3ZnSO
4+3Na
2CO
3+4H
2O=ZnCO
3?2Zn��OH��
2?2H
2O��+3Na
2SO
4+2CO
2����
�ʴ�Ϊ��3ZnSO
4+3Na
2CO
3+4H
2O=ZnCO
3?2Zn��OH��
2?2H
2O��+3Na
2SO
4+2CO
2����
�ۼ������պ�������Ƿ���̼������ɣ�ʵ�����Ϊ��ȡ�������յĹ��壬����ϡ���ᣬ�۲��Ƿ������ݲ������������ݲ�����˵������ȫ�ֽ⣬
�ʴ�Ϊ��ȡ�������յĹ��壬����ϡ���ᣬ�۲��Ƿ������ݲ������������ݲ�����˵������ȫ�ֽ⣮
��������1�����ӽṹ��H
2O
2���ƣ�S
2Cl
2��չ����ҳ�ͽṹ��Cl-Sλ��������ҳ���ڣ�S
2Cl
2������Sԭ��֮���γ�1�Թ��õ��Ӷԣ�Clԭ����Sԭ��֮���γ�1�Թ��õ��Ӷԣ�
���ӽṹ��H
2O
2���ƣ����ڷ��Ӿ��壻
��2��S
2Cl
2��ˮ��ˮ�⣬��������ʹƷ����Һ��ɫ�����壬������Ϊ���������ڷ�Ӧ��������Ԫ��һ�������ߵ�+4�ۣ�����SO
2����һ���ֽ��͵�0�ۣ�����S����ͬʱ����HCl��
��1�����ɹ������̿�֪�����������������������������������ɣ�Ӧ�Ǹ���������������������������������������̣���������������������ɶ������̣����ݿ��Ƶ�PHֵ��ԭ���غ㡢����غ��֪������ˮ�μӷ�Ӧ��ͬʱ�����������ӣ�
�����̢��м��������Ŀ�ģ�Ӧ�dz�ȥ��Һ�н������ӣ��ֲ����������ʣ�Ӧ��ѡ��п��
��п����ˮ�⣬���̢��е�pH=1Ŀ��������Zn
2+��ˮ�⣻
��2�������չ��壬Ӧ��������
�ڷ�Ӧ����ZnCO
3?2Zn��OH��
2?2H
2O�����ɵ�����Ϊ������̼������Ԫ���غ��֪���������������ɣ�
�ۼ������պ�������Ƿ���̼������ɣ��������ᷴӦ���������жϣ�
���������⿼�黯ѧ�����������̡�����Ϣ����ȡ���õȣ���Ŀ�ѶȽϴ����ҵ�����ǽ���Ĺؼ���ע��������ʵ�������������ԭ������Ҫѧ��������ʵ�Ļ���������֪ʶ���ӽ�������������

 ��
��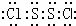 �����Ӿ��壻
�����Ӿ��壻

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
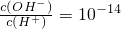 ����Һ�У�Al3+��NH4+��Cl-��CO32-
����Һ�У�Al3+��NH4+��Cl-��CO32-