
��80��ʱ����0.4mol�������������������2L�ѳ�յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
ʱ�䣨s�� C��mol/L�� | 0 | 20 | 40 | 60 | 80 | 100 |
C��N2O4�� | 0. 20 | a | 0.10 | c | d | e |
C��NO2�� | 0.00 | 0.12 | b | 0.22 | 0.22 | 0.22 |
��Ӧ������100s��Ӧ�������¶Ƚ��ͣ������������ɫ��dz��
��1���÷�Ӧ�Ļ�ѧ����ʽΪ__________________________������b_________c���<������=������>������
��2��20sʱ��N2O4�ĵ�Ũ��Ϊ__________________mol��L��1��0��20s��N2O4��ƽ����Ӧ����Ϊ________________��
��3���÷�Ӧ��ƽ�ⳣ������ʽK=___________________
��80��ʱ�÷�Ӧ��ƽ�ⳣ��KֵΪ:______________��������С�����2λ����
��4��������������ͬʱ���÷�Ӧ��KֵԽ��������ƽ��ʱ____________��
A.N2O4��ת����Խ�� B.NO2�IJ���Խ��
C.N2O4��NO2��Ũ��֮��Խ�� D.����Ӧ���еij̶�Խ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ����3�¸߿���Ӧ�Բ������ۻ�ѧ�Ծ��������棩 ���ͣ������
��ˮMgBr2������������ʵ���Ҳ���þм��Һ��Ϊԭ���Ʊ���ˮMgBr2��װ������ͼ��ʾ���г�������ȥ������Ҫ�������£�

����l������ƿ��װ��10gþм��150mL��ˮ���ѣ�װ��B�м���������Һ�塣
����2������ͨ�����ĵ�����ֱ������ȫ��������ƿ�С�
����3����Ӧ��Ϻ�ָ������£����ˣ�����Һת������һ�������ƿ�У���ȴ��0�棬�������壬�ٹ��˵������Ѻ��廯þ��Ʒ��
����4���������ñ��ܽ��Ʒ����ȴ��0�棬�������壬���ˣ�ϴ�ӵ������Ѻ��廯þ��������160��ֽ����ˮMgBr2��Ʒ��
��֪����Mg��Br2��Ӧ���ҷ��ȣ�MgBr2����ǿ��ˮ�ԡ�
��MgBr2+3C2H5OC2H5= MgBr2��3C2H5OC2H5
��ش�
(1)����A��������___________������ʵ���е�������____________��
(2)����2�У����Խ�Bװ���е�����ȫ��������ƿ�е�ԭ����_______����ʵ�����������Һ��һ����ȫ����������ƿ�У�������_____________��
(3)����3�У���һ�ι��˵õ��Ĺ���������______�����ݲ���3��4�����ܽ�������Ѻ��廯þ�����е��������ʣ�_________��
(4)�����Mg���������ʵ��֤��O2�������Ա�N2��ǿ��________��
(5)������õ��IJ�Ʒ�ڸ���������ȴ�����º�����������Ϊ61.4g�����ʵ����ȡMgBr2�IJ�����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016~2017ѧ�꽭��ʡ��Ǩ�и߶�ѧҵˮƽ����ģ�⣨������ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ��װ�ò��ܴﵽʵ��Ŀ�ĵ���
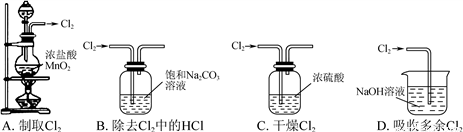
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���У��ݣ�������һ�����ϵ�����3�������������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
��ҵ����ú��ˮΪԭ��ͨ��һϵ��ת���ɱ�Ϊ�����Դ������ҵԭ�ϼ״���
��1����֪��C(s)+O2(g)=CO2(g) ��H1
��2H2(g)+O2(g)=2H2O (l) ��H2
��H2O (l)= H2O (g) ��H3
��̼��ˮ������ӦC(s)+2H2O(g) CO2(g)+2H2(g)�Ħ�H =________��
CO2(g)+2H2(g)�Ħ�H =________��
��2����ҵ��Ҳ���Խ�����������Ӧ�õ���CO2��H2��һ���ϳɼ״�����Ӧ����ʽΪ��CO2(g)��3H2(g) CH3OH(g)��H2O(g����H��0
CH3OH(g)��H2O(g����H��0
�ٹ�ҵ����������CO2��H2��ת����________���ǰ�ߴ������ߴ���һ�������жϡ�����Ϊ����״��IJ��ʿ��Բ�ȡ�Ĵ�ʩ��_______________�������㣩��
����һ���º����ܱ������г���1 mol CO2��3 mol H2����������Ӧ�����CO2��CH3OH(g)Ũ����ʱ��仯����ͼ��ʾ�����¶��µ�ƽ�ⳣ��Ϊ______��������λ��Ч���֣���
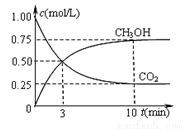
�ı��¶ȣ�ʹ��ӦCO2(g)+3H2(g) CH3OH(g)+H2O(g)�е��������ʶ�Ϊ��̬����ʼ�¶������ͬ��T1�桢2 L�ܱ�����������Ӧ�����в������ݼ��±���
CH3OH(g)+H2O(g)�е��������ʶ�Ϊ��̬����ʼ�¶������ͬ��T1�桢2 L�ܱ�����������Ӧ�����в������ݼ��±���
��Ӧʱ�� | CO2��mol�� | H2��mol�� | CH3OH��mol�� | H2O��mol�� | |
��Ӧ�� ���º��� | 0min | 2 | 6 | 0 | 0 |
10min | 4.5 | ||||
20min | 1 | ||||
30min | 1 | ||||
��Ӧ�� ���Ⱥ��� | 0min | 0 | 0 | 2 | 2 |
�ٴﵽƽ��ʱ����Ӧ��Աȣ�ƽ�ⳣ��K(��)___K(��)�����������������=������ͬ����ƽ��ʱCH3OH��Ũ��c(��)___c(��)��
�ڶԷ�Ӧ��ǰ10 min�ڵ�ƽ����Ӧ���ʦ�(CH3OH)=______����30 minʱֻ���������ٳ���1 mol CO2(g)��1 mol H2O(g)����ƽ��_____�ƶ��������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���У��ݣ�������һ�����ϵ�����3�������������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
���й����л����������ȷ����
A. ��ϩ��������ϩ�ͱ������о�����̼̼˫��
B. ������֬������ʹ����KMnO4��Һ��ɫ
C. �ȱ�����������ԭ�Ӷ�����ͬһƽ��
D. �ױ������ϵ�һ����ԭ�ӱ���C3H6Clȡ�����γɵ�ͬ���칹����9��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡտ���и߶���ѧ����ĩ���п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪�����£�KSP(AgCl)=1.8��10-10 mol2��L-2��KSP(AgI)=8.3��10-17mol2��L-2�����������У� ��ȷ����
A. �����£�AgCl �ڱ��� NaCl ��Һ�е� KSP ���ڴ�ˮ�е� KSP С
B. �� AgCl ������Һ�м��� KI ��Һ�������ɰ�ɫת��Ϊ��ɫ
C. �� AgCl �ı�����Һ�м��� NaCl ���壬�� AgCl ��������Һ�� c(Ag+)=c(Cl-)
D. �� 0.001 mol��L-1 �� AgNO3 ��Һ���� KCl �� KI �Ļ����Һ�У�һ���Ȳ��� AgI ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡտ���и߶���ѧ����ĩ���п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
������ʵ�����õ绯ѧ���۽��͵���
A. �ִ�ˮ�����µĴ�����װһ��������п��
B. ��Ƭ�������ⷽ������
C. ��п��ϡ���ᷴӦʱ��������������ͭ��Һ�����ʼӿ�
D. ��п�����ȶ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ij�¶��£���ͬpH������ʹ�����Һ�ֱ��ˮϡ�ͣ�ƽ��pH����Һ����仯��������ͼ��ʾ����ͼ�ж���ȷ����
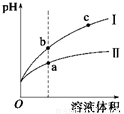
A. ��Ϊ����ϡ��ʱ��pH�仯����
B. ��Һ�ĵ����ԣ� a > b > c
C. a��KW����ֵ��c��KW����ֵ��
D. b�������Ũ�ȴ���a�������Ũ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�żҿ��и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��ҵ�ϣ���500�����ҵ���м��ͨ��Cl2������ˮ�Ȼ��������Ʊ������о�Ҫȷ����ˮ����ģ��ù�����ͼʾװ�ý���ʵ�飺
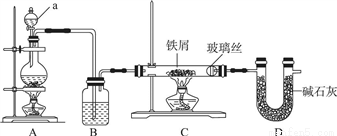
��1������a������Ϊ________��װ��A��Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ________��Ϊ����װ��CΪ��ˮ������װ��B�м�����Լ���________��
��2��ʵ�鲽�裺��ͼ����װ�ú���________����ʵ�����������װҩƷ��Ȼ���ȼ________���A����C�������ƾ��ƣ���________����ʵ������ʱ���ٵ�ȼ________���A����C�������ƾ��ơ�
��3��װ��D��������________��________��
��4����ͬѧ��Ϊ����װ��C�������л���HCl��Ӧ��װ��Bǰ����װ��________�����Լ����ƣ���ϴ��װ�ó�ȥ����ͬѧ��Ϊ����Ҫ��ȥHCl������Ϊ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com