
�������ö��Ե缫���200 mL һ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ����ͼ��ʾ��������������ѻ���ɱ�״���µ������������ͼ����Ϣ�ش��������⡣
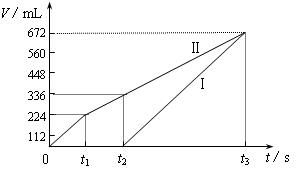
��ͨ�������Ʋ⣺
��ԭ�����ҺNaCl��CuSO4�����ʵ���Ũ�ȣ�
��t2ʱ������Һ��pH��
�����ö��Ե缫���NaCl��CuSO4�Ļ����Һ200mL������һ��ʱ����������õ�224mL���壬��ԭ�����Һ�е�������Ũ�ȵ�ȡֵ��ΧΪ ��ͭ����Ũ�ȵ�ȡֵ��ΧΪ ��
�Ţ����������ݳ�����Cl2
n(NaCl)��2n(Cl2)��0.02 mol����c(NaCl)��0.1mol/L ��1�֣�
�����õ�336 mL�����У���0.01 mol Cl2��0.005 mol O2
ת�Ƶ��ӵ����ʵ���Ϊ��0.01 mol��2��0.005 mol��4��0.04 mol
�˹����������պ�ȫ������ͭ
n(CuSO4)��n(Cu)��0.04 mol��2��0.02 mol
��c(CuSO4)��![]() ��0.1mol/L ��2�֣�
��0.1mol/L ��2�֣�
��t2ʱ��Һ��c(Na��)��0.1 mol/L��c(SO42��)��0.1 mol/L
���ݵ���غ��У�c(H��)��2��0.1 mol/L��0.1 mol/L��0.1 mol/L
����Һ��pH��1 ��2�֣�
��0��c(Cl��)��0.1mol/L��2�֣� 0��c(Cu2��)��0.05mol/L��2�֣�


 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڳ������ö��Ե缫���2 L����ʳ��ˮ���缫��ͨ��0.2 mol ����ʱֹͣ��⣬��ʱ������ɵ�NaOH�����Ƕ��٣���Һ��pH�Ƕ��٣�����Һ������仯���Բ��ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�����еڰ���ѧ2010������꼶������¿���ѧ���� ���ͣ�������
�������ö��Ե缫���200 mL һ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ����ͼ��ʾ��������������ѻ���ɱ�״���µ������������ͼ����Ϣ�ش��������⡣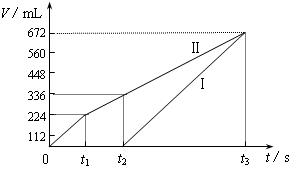
��ͨ�������Ʋ⣺
��ԭ�����ҺNaCl��CuSO4�����ʵ���Ũ�ȣ�
��t2ʱ������Һ��pH��
�����ö��Ե缫���NaCl��CuSO4�Ļ����Һ200mL������һ��ʱ����������õ�224mL���壬��ԭ�����Һ�е�������Ũ�ȵ�ȡֵ��ΧΪ ��ͭ����Ũ�ȵ�ȡֵ��ΧΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�찲�����������ѧ������ѧ��ʵ�����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ö��Ե缫���1L����KCl����Һ����ͨ��n mol���ӵĵ���������Һ��pH��n�Ĺ�ϵ�ǣ�����ǰ����Һ��������䣩�� ��
A��pH=n B��pH=��lg n C��pH=14��lgn D��pH=lgn+14
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com