
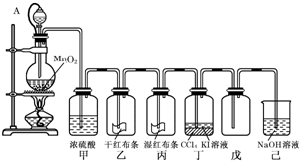
��10�֣�Ϊ���ڡ����������ʺ���;����ij��ʦ���ö������̺�Ũ����Ϊ��Ҫԭ�ϣ����һ����ͼ��ʾ��ʵ��װ��(����A������ע������ͷ�� ��Ƥ�ܣ���ͷ�Ѳ��벢������Ƥ��)���н�ѧ���Իش��������⣺
��Ƥ�ܣ���ͷ�Ѳ��벢������Ƥ��)���н�ѧ���Իش��������⣺

��1��������ƿ������Ӧ�Ļ�ѧ����ʽΪ ��
��2����������� ��
���е����ӷ���ʽ��______________________________����
��3��������Ƥ�ܽ���Һ©���϶�����ƿ��ͨ���������� ��
��4����֪��������ˮ��Ӧ�Ļ�ѧ����ΪCl2 + H20 =" HCl" + HClO��
������Ϊʹ��ɫ������ɫ�������� ��
��Ϊ̽�����������Ư����,����Ƽ�ʵ�����֤����д��ʵ��IJ�������������ͽ���
 �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
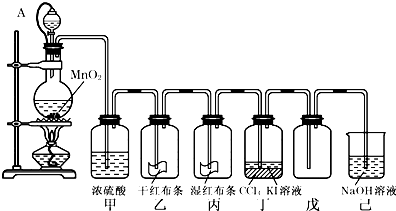
Ϊ���ڡ����������ʺ���;����ij��ʦ���ö������̺�Ũ����Ϊ��Ҫԭ�ϣ����һ����ͼ��ʾ��ʵ��װ�ã�����A������ע������ͷ����Ƥ�ܣ���ͷ�Ѳ��벢������Ƥ���������˽�ѧ���Իش��������⣺
��1�����е��������������������������������е�������������������������������������Һ��ķ�����____________��
��2����Ƥ����ͨ��Һ©���е�����ѹǿp1����ƿ������ѹǿp2�Ĺ�ϵΪ��
p1_____________p2������ڡ�����С�ڡ����ڡ�����������Ƥ�ܵ�Ŀ����
___________________________ ____________________��
��3����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ��__________________________________��
���з��������ӷ���ʽ��____________________ _ _________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
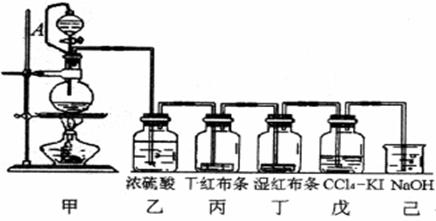
Ϊ���ڡ����������ʺ���;����ij��ʦ���ö������̺�Ũ����Ϊ��Ҫԭ�ϣ����һ����ͼ��ʾ��ʵ��װ��(����A������ע������ͷ����Ƥ�ܣ���ͷ�Ѳ��벢������Ƥ��)���н�ѧ���Իش��������⣺

��1��������ƿ������Ӧ�Ļ�ѧ����ʽΪ ��
��2����������� ��
���е����ӷ���ʽ��______________________________����
��3��������Ƥ�ܽ���Һ©���϶�����ƿ��ͨ���������� ��
��4����֪��������ˮ��Ӧ�Ļ�ѧ����ΪCl2 + H20 = HCl + HClO��
������Ϊʹ��ɫ������ɫ�������� ��
��Ϊ̽�����������Ư����,����Ƽ�ʵ�����֤����д��ʵ��IJ�������������ͽ���![]()
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com