

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��һ����Һ��H+��OH-�⣬����Na+��![]() ��Cl-������������ӵ�Ũ�ȷֱ���0.01 mol��L-1��0.003 5 mol��L-1��0.004 mol��L-1������Һ��pHΪ__________��
��Cl-������������ӵ�Ũ�ȷֱ���0.01 mol��L-1��0.003 5 mol��L-1��0.004 mol��L-1������Һ��pHΪ__________��
(2)NH4Cl��Һ�����ԣ��������ӷ���ʽ��ʾ��һ��Ӧ��___________________________��
(3)Һ��������ˮ�ĵ��룬��д��Һ���ĵ��뷽��ʽ��______________________________����Һ���м���NH4Cl����ƽ�⽫��____________�ƶ���
(4)pH��ͬ�İ�ˮ������������Һ���ֱ�������ˮϡ����ԭ���������m����n����ϡ�ͺ�����Һ��pH����ͬ����m____________n(ѡ�������������=��)��������pH��ͬ�������������Һ������������������m____________n(ѡ�������������=��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ش���������:
(1)��һ����Һ��H+��OH-�⣬����Na+��SO42-��Cl-������������ӵ�Ũ�ȷֱ���0.01 mol��L-1��0.0035 mol��L-1��0.004 mol��L-1������Һ��pHΪ ��
(2)Һ��������ˮ�ĵ��룬��д��Һ���ĵ��뷽��ʽ ��
��3���������ӷ���ʽ��д��ȷ���� �� ?
A��ʯ������Na2CO3��Һ��ϣ�Ca2++CO32��=====CaCO3��?
B��NH4HSO3��Һ��������NaOH��Һ��ϼ��ȣ�
NH4+ + HSO3- +2OH-![]() NH3��+SO32- +2H2O?
NH3��+SO32- +2H2O?
C������������KIO3��Һ��KI��Һ��Ӧ����I2�� IO32- +5I-+3H2O====3I2+6OH-
D��AgNO3��Һ�м��������ˮ��Ag++NH3��H2O=====AgOH��+NH4+
E��H2SO4��Ba(OH)2��Һ��Ӧ��Ba2++OH-+H++SO42-===== BaSO4��+H2O?
F��CuSO4��Һ����H2S���壺Cu2++H2S======CuS��+2H+?
G��AlCl3��Һ�м��������Ũ��ˮ��Al3++4NH3��H2O ===== AlO2- +4NH 4+ +2H2O
H�����������Ũ�ȵ�Ba(OH)2ϡ��Һ��NH4HCO3ϡ��Һ��ϣ�
Ba2++2OH-+NH4+ +HCO3-=== BaCO3��+NH3��H2O+H2O
I��LiAlH4��������ˮ��õ���ɫ��Һ����ѧ����ʽ�ɱ�ʾΪ��
LiAlH4+2H2O ===LiAlO2 +4H2��
��4��Na2O2��HCl��Al2O3����������ˮ����ȫ��Ӧ����Һ��ֻ����Na+��H+��Cl-��OH-������Һ�����ԣ���Na2O2��HCl��Al2O3�����ʵ���֮�ȿ���Ϊ ��
A.3��2��1 B.2��4��1? C.2��3��1 D.4��2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ش���������:
(1)��һ����Һ��H+��OH-�⣬����Na+��SO42-��Cl-������������ӵ�Ũ�ȷֱ���0.01 mol��L-1��0.0035 mol��L-1��0.004 mol��L-1������Һ��pHΪ ��
(2)Һ��������ˮ�ĵ��룬��д��Һ���ĵ��뷽��ʽ ��
��3���������ӷ���ʽ��д��ȷ���� �� ?
A��ʯ������Na2CO3��Һ��ϣ�Ca2++CO32��=====CaCO3��?
B��NH4HSO3��Һ��������NaOH��Һ��ϼ��ȣ�
NH4+ + HSO3- +2OH- NH3��+SO32- +2H2O?
C������������KIO3��Һ��KI��Һ��Ӧ����I2�� IO32- +5I-+3H2O====3I2+6OH-
D��AgNO3��Һ�м��������ˮ��Ag++NH3��H2O=====AgOH��+NH4+
E��H2SO4��Ba(OH)2��Һ��Ӧ��Ba2++OH-+H++SO42-===== BaSO4��+H2O?
F��CuSO4��Һ����H2S���壺Cu2++H2S======CuS��+2H+?
G��AlCl3��Һ�м��������Ũ��ˮ��Al3++4NH3��H2O ===== AlO2- +4NH 4+ +2H2O
H�����������Ũ�ȵ�Ba(OH)2ϡ��Һ��NH4HCO3ϡ��Һ��ϣ�
Ba2++2OH-+NH4+ +HCO3-=== BaCO3��+NH3��H2O+H2O
I��LiAlH4��������ˮ��õ���ɫ��Һ����ѧ����ʽ�ɱ�ʾΪ��
LiAlH4+2H2O ===LiAlO2 +4H2��
��4��Na2O2��HCl��Al2O3����������ˮ����ȫ��Ӧ����Һ��ֻ����Na+��H+��Cl-��OH-������Һ�����ԣ���Na2O2��HCl��Al2O3�����ʵ���֮�ȿ���Ϊ ��
A.3��2��1 B.2��4��1? C.2��3��1 D.4��2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���㽭ʡ��ʮ���и�����ѧ��11���¿���ѧ�� ���ͣ������
�ش���������:
(1)��һ����Һ��H+��OH-�⣬����Na+��SO42-��Cl-������������ӵ�Ũ�ȷֱ���0.01 mol��L-1��0.0035 mol��L-1��0.004 mol��L-1������Һ��pHΪ ��
(2)Һ��������ˮ�ĵ��룬��д��Һ���ĵ��뷽��ʽ ��
��3���������ӷ���ʽ��д��ȷ���� �� ?
A��ʯ������Na2CO3��Һ��ϣ�Ca2++CO32��=====CaCO3��?
B��NH4HSO3��Һ��������NaOH��Һ��ϼ��ȣ�
NH4+ + HSO3- +2OH-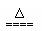 NH3��+SO32- +2H2O?
NH3��+SO32- +2H2O?
C������������KIO3��Һ��KI��Һ��Ӧ����I2�� IO32- +5I-+3H2O====3I2+6OH-
D��AgNO3��Һ�м��������ˮ��Ag++NH3��H2O=====AgOH��+NH4+
E��H2SO4��Ba(OH)2��Һ��Ӧ��Ba2++OH-+H++SO42-="====" BaSO4��+H2O?
F��CuSO4��Һ����H2S���壺Cu2++H2S======CuS��+2H+?
G��AlCl3��Һ�м��������Ũ��ˮ��Al3++4NH3��H2O ="====" AlO2- +4NH 4+ +2H2O
H�����������Ũ�ȵ�Ba(OH)2ϡ��Һ��NH4HCO3ϡ��Һ��ϣ�
Ba2++2OH-+NH4+ +HCO3-="== " BaCO3��+NH3��H2O+H2O
I��LiAlH4��������ˮ��õ���ɫ��Һ����ѧ����ʽ�ɱ�ʾΪ��
LiAlH4+2H2O ="==LiAlO2" +4H2��
��4�� Na2O2��HCl��Al2O3����������ˮ����ȫ��Ӧ����Һ��ֻ����Na+��H+��Cl-��OH-������Һ�����ԣ���Na2O2��HCl��Al2O3�����ʵ���֮�ȿ���Ϊ ��
Na2O2��HCl��Al2O3����������ˮ����ȫ��Ӧ����Һ��ֻ����Na+��H+��Cl-��OH-������Һ�����ԣ���Na2O2��HCl��Al2O3�����ʵ���֮�ȿ���Ϊ ��
A.3��2��1 B.2��4��1? C.2��3��1 D.4��2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���㽭ʡ������ѧ��11���¿���ѧ�� ���ͣ������
�ش���������:
(1)��һ����Һ��H+��OH-�⣬����Na+��SO42-��Cl-������������ӵ�Ũ�ȷֱ���0.01 mol��L-1��0.0035 mol��L-1��0.004 mol��L-1������Һ��pHΪ ��
(2)Һ��������ˮ�ĵ��룬��д��Һ���ĵ��뷽��ʽ ��
��3���������ӷ���ʽ��д��ȷ���� �� ?
A��ʯ������Na2CO3��Һ��ϣ�Ca2++CO32��=====CaCO3��?
B��NH4HSO3��Һ��������NaOH��Һ��ϼ��ȣ�
NH4+ + HSO3- +2OH-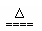 NH3��+SO32- +2H2O?
NH3��+SO32- +2H2O?
C������������KIO3��Һ��KI��Һ��Ӧ����I2�� IO32- +5I-+3H2O====3I2+6OH-
D��AgNO3��Һ�м��������ˮ��Ag++NH3��H2O=====AgOH��+NH4+
E��H2SO4��Ba(OH)2��Һ��Ӧ��Ba2++OH-+H++SO42-===== BaSO4��+H2O?
F��CuSO4��Һ����H2S���壺Cu2++H2S======CuS��+2H+?
G��AlCl3��Һ�м��������Ũ��ˮ��Al3++4NH3��H2O ===== AlO2- +4NH 4+ +2H2O
H�����������Ũ�ȵ�Ba(OH)2ϡ��Һ��NH4HCO3ϡ��Һ��ϣ�
Ba2++2OH-+NH4+ +HCO3-=== BaCO3��+NH3��H2O+H2O
I��LiAlH4��������ˮ��õ���ɫ��Һ����ѧ����ʽ�ɱ�ʾΪ��
LiAlH4+2H2O ===LiAlO2 +4H2��
��4��Na2O2��HCl��Al2O3����������ˮ����ȫ��Ӧ����Һ��ֻ����Na+��H+��Cl-��OH-������Һ�����ԣ���Na2O2��HCl��Al2O3�����ʵ���֮�ȿ���Ϊ ��
A.3��2��1 B.2��4��1? C.2��3��1 D.4��2��1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com