
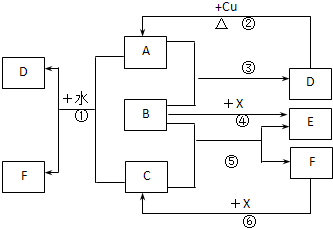
��16�֣�A��F����ѧ��ѧ�г������ʣ�������A��C��E��FΪ���壬B��DΪҺ�壬����B�ķ���Ϊ4ԭ�ӷ��ӣ�D�ڳ����²����лӷ��ԡ�F��Ũ��Һ��X����ͨ������ʵ�����Ʊ�����C��X��һ�ֺ�ɫ��ĩ����Щ����֮��������ͼ��ʾ��ת����ϵ��ͼ�в�������������ȥ��
��1��д���������ʵĻ�ѧʽ��A ��F ��
��2��B�ĵ���ʽΪ ������ͼ����Ϣ��B��C��X����������ǿ������˳���� ���û�ѧʽ��ʾ����
��3��д����Ӧ�ٵ����ӷ���ʽ ��
д����Ӧ�����ӷ���ʽ ��
��4���ڷ�Ӧ���У�F���ֵ������� ��������0.75 mol Cʱ���������Ļ�ԭ�������ʵ����� ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
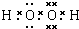
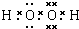
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
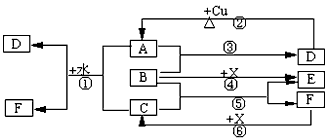
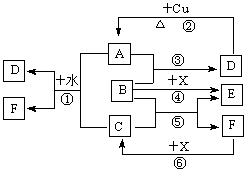 (�����7��)��֪A��F����ѧ��ѧ�г������ʣ�����A��C��E��FΪ���壬B��DΪҺ�壬D������������Ϊһ�����ҹ�ҵ����ˮƽ��һ�ֱ�־��ʵ������B��E����F��Ũ��Һ������Cʱ���õ���ɫ��ĩX��B��������18�����ӡ���Ӧ�в�������������ȥ���Իش��������⣺
(�����7��)��֪A��F����ѧ��ѧ�г������ʣ�����A��C��E��FΪ���壬B��DΪҺ�壬D������������Ϊһ�����ҹ�ҵ����ˮƽ��һ�ֱ�־��ʵ������B��E����F��Ũ��Һ������Cʱ���õ���ɫ��ĩX��B��������18�����ӡ���Ӧ�в�������������ȥ���Իش��������⣺
(1)B�ĽṹʽΪ________________________________��
(2)д����ѧ����ʽ����_____________________________________________����____________________________________________________
(3)д�����ӷ���ʽ����_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008-2009ѧ���㽭ʡ�����а�У������һ���£���ĩ��ѧ�Ծ��������棩 ���ͣ������
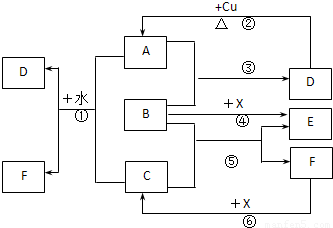
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com