
����Ŀ��ij�л�������A�����ϣ��������к�̼Ϊ70.6%������Ϊ5.9%���ຬ�������������з����ⶨ���л����������Է��������ͷ��ӽṹ��
����һ:������������֪A������ͼ��ͼ�£�
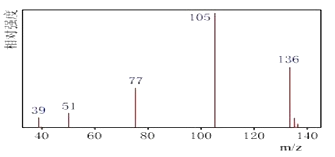
������: �˴Ź����Dz��A�ĺ˴Ź���������ͼ��
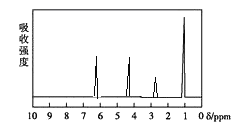
������: ���ú�������Dz��A���ӵĺ�����ף�����ͼ��
��֪��A�����б�����ֻ��һ��ȡ�������Իش��������⡣
��1��A���ӵ���Է�������_______________������____________�ֻ�ѧ������ͬ����ԭ�ӡ�
��2��A�ķ���ʽΪ_______________________��
��3�����л��ﺬ�еĹ�������______________________��
��4��A�ķ�����ֻ��һ������ֱ��������________________(�����)��
a��A����Է������� b��A�ķ���ʽ
c��A�ĺ˴Ź�������ͼ d��A���ӵĺ������ͼ
��5��A�Ľṹ��ʽΪ_______________________________��
���𰸡�136 4 C8H8O2 ���� bc ![]()
��������
��1����A������ͼ��֪��A����Է�����Ϊ136����A�ĺ˴Ź�������ͼ��֪��A��4���壬��A����4�ֻ�ѧ������ͬ����ԭ�ӣ��ʴ�Ϊ��136��4��
��2��N(C)��N(H)��N(O)=70.6%/12��5.9%/1��(1-70.6%-5.9%)/16=4��4��1����A��ʵ��ʽΪC4H4O����A�ķ���ʽΪ(C4H4O)n����Mr(A)=136���ɵ�n=2����A�ķ���ʽΪ��C8H8O2��
�ʴ�Ϊ��A�ķ���ʽΪ��C8H8O2��
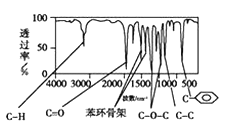
��3����A���ӵĺ������֪��A���б�����ռ6��Cԭ����������C=O��C-O-C��C-C��C-H������C=O��C-O-C�����Ϊ![]() �����Ը�����Ϊ������
�����Ը�����Ϊ������
�ʴ�Ϊ��������
��4�����������2���Ľ������ٽ��A���ӵĺ˴Ź������ף���4����ԭ�ӣ��������Ϊ1��1��1��3��������-CH3����ԭ����Ϊ3������ֻ����һ��-CH3������A�ķ�����ֻ��һ����������Ϊbc���ʴ�Ϊ��bc��
(5)��A���ӵĺ������ͼ��֪��A�����к���һ������������һ��������ֻ����һ�������������Ϸ�����֪��A�Ľṹ��ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�����Ļ���������ܱ������з�����Ӧ m A (g) + n B (g) ![]() p C (g)�ﵽƽ����¶Ȳ��䣬�����������С��ԭ����1/2���ﵽƽ��ʱ��C��Ũ��Ϊԭ����1.5����������˵����ȷ����
p C (g)�ﵽƽ����¶Ȳ��䣬�����������С��ԭ����1/2���ﵽƽ��ʱ��C��Ũ��Ϊԭ����1.5����������˵����ȷ����
A. m + n > pB. C�������������
C. ƽ��������Ӧ�����ƶ�D. A ��ת���ʽ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ü�������������ȡ����Ӧ�ĸ���Ʒ��������������ڹ�ҵ���ѳ�Ϊ��ʵ��ij��ѧ��ȤС��ģ���������̣�����Ƶ�װ����ͼ��ʾ��
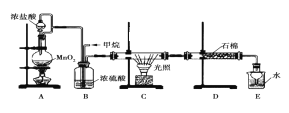
(1)A����ȡCl2�ķ�Ӧ�Ļ�ѧ����ʽ��_________________________��
(2)Bװ�������ֹ��ܣ��ٿ��������ٶȣ��ھ��Ȼ�����壻��_______________________��
(3)Dװ���е�ʯ���������ų�ʪ��KI����������________________��
(4)Eװ�õ�������_______________________��
(5)Eװ���г��������������⣬�������л����E�з�����������ѷ�����______________��
(6)��1 mol CH4��Cl2����ȡ����Ӧ�����4���л�ȡ����������ʵ�����ȣ������ĵ����������ʵ�����_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵����ȷ����
A.22.4LCl2��������ˮ��������Һ��Cl2��Cl-��HClO��ClO-����������ΪNA
B.��״���£�38g3H2O2�к���3NA���ۼ�
C.�����£���5.6g����Ͷ������Ũ�����У�ת��0.3NA����
D.��״���£�5.6L һ��������5.6L ������Ϻ�ķ�������Ϊ0.5NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��WΪ������Ԫ�أ���Wԭ�ӵ����������������ڲ���ӵ�![]() ����ͼ��ʾ������˵������ȷ����
����ͼ��ʾ������˵������ȷ����
X | Y | |
Z | W |
A.XԪ�ص��⻯����Ӽ�����γ����
B.YԪ�ص�����ͬ�������峣���¶�������
C.�����Ӱ뾶�Ӵ�С��˳��ΪX>Y>Z>W
D.����������Ӧ��ˮ��������ԣ�W>Z
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�ǻ�ѧ����С����Ƶ��û�ѧ��ԴʹLED�Ʒ����װ��ʾ��ͼ�������йظ�װ�õ�˵����ȷ����( )

A. ͭƬΪ�������丽������Һ��������Һ����Cu2������
B. �����пƬ������Ƭ����·�еĵ������ı�
C. ������ת������ʽ��Ҫ������ѧ����������������
D. �����ϡ���ỻ������֭��LED�ƽ����ᷢ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij�¶�ʱ����һ�� 10L �ĺ��������У�X��Y��Z ��Ϊ���壬�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ������ͼ��������գ�
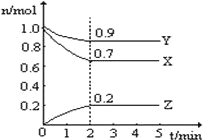
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ��________________________��
(2)��Ӧ��ʼ�� 2min�������� Z ��ʾ��ƽ����Ӧ����Ϊ��____________________��
(3)�� a mol X �� b mol Y �Ļ�����巢��������Ӧ����Ӧ��ijʱ�̸����ʵ���ǡ�����㣺n(X)=n(Y)=2n(Z)����ԭ��������� a��b=________��
���ں��º��ݵ��ܱ������У����������������ٷ����仯ʱ��
�ٻ�������ѹǿ���ڻ��������ܶȣ��ۻ������������ʵ������ܻ�������ƽ����Է����������ݻ���������ɫ������Ӧ���������ķ�Ӧ����֮�ȵ��ڻ�ѧ������֮�ȡ�
(1)һ����֤��2SO2(g)+O2(g) 2SO3(g)�ﵽƽ��״̬���� __________________����ţ���ͬ)��
(2)һ����֤�� I2(g)+H2(g)2HI(g)�ﵽƽ��״̬����____________________��
(3)һ����֤�� A(s)+2B(g)C(g)+D(g)�ﵽƽ��״̬����_________________(B��C��D ����ɫ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���ż��������ơ���У��Ǿ��������һ��������Ҫ��Ʒ�֣�ij�о���ѧϰС��Ϊȷ����ż���Ľṹ����������̽����
����һ������ż������ͨ���ȵ�����ͭ(����)�����ɶ�����̼��ˮ������װ����ˮ�Ȼ��ƺ����������Ƶ����չ���ȫ���գ���ͼ1��2.64 g��ż����������������5.28 g������̼��2.16 gˮ��
�����������ʹ��ż�������������ܶ�����ͬ������H2��44��
���������ú˴Ź����Dz����ż���ĺ˴Ź���������ͼ2��ͼ��4����������Ϊ1��3��1��3��
�����ģ����ú�������Dz����ż�����ӵĺ��������ͼ3��



��1��ͼ1װ������֧U�ܲ��ܻ�����������__________________________��
��2����ż����Ħ������Ϊ____________��
��3����ż���ķ���ʽΪ____________________��
��4����ż���Ľṹ��ʽΪ ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����þ����һ�ֹ�ҵ���ϣ���Ҫ�ɷ���MgO��ռ40%������������������CaO��MnO��Fe2O3��FeO��Al2O3��SiO2�����ʣ��Դ�Ϊԭ����ȡ������þ��������ӡȾ����ֽ��ҽҩ�ȹ�ҵ������þ������ȡMgSO47H2O�Ĺ����������£�
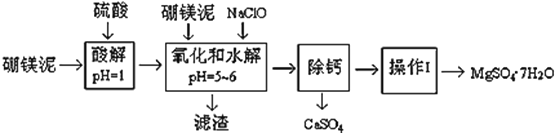
��1��ʵ������Ҫ1 mol/L������800 mL������ 98% ��Ũ���ᣨ��= 1.84 g/mL�������ƣ���ȡŨ������Ҫʹ����Ͳ�Ĺ��Ϊ__________����дѡ����ĸ��
A��10 mL B��20 mL C��50 mL D��100 mL
��2�������NaClO����Mn2+ ��Ӧ��Mn2+ + ClO + H2O = MnO2��+ 2H+ + Cl ���ڸò����л���һ������Ҳ�ᱻNaClO�������÷�Ӧ�����ӷ���ʽΪ___________________��
��3����������Ҫ�ɷֳ�����Fe(OH)3��Al(OH)3�⣬������__________��___________��
��4���ڡ����ơ�ǰ���������Һ��Fe3+ �Ƿ������������鷽��___________________����д������������ͽ��ۣ�
��5����֪MgSO4��CaSO4 ���ܽ�ȣ���λΪ g/100 g ˮ�����±���
�¶ȣ��棩 | 40 | 50 | 60 | 70 |
MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
�����ơ��ǽ�MgSO4��CaSO4�����Һ�е�CaSO4��ȥ�������ϱ����ݣ���Ҫ˵����������______�����������ǽ���Һ��������Ũ������ȴ�ᾧ��______����õ���MgSO47H2O
��6��ʵ�����ṩ����þ�100 g���õ� MgSO47H2OΪ172.2 g ����MgSO47H2O �IJ���Ϊ___��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com