
 ����ͼ���������Ա�Ҫ�����Ӳ���գ�
����ͼ���������Ա�Ҫ�����Ӳ���գ� H2��+2NaOH+Cl2��
H2��+2NaOH+Cl2�� H2��+2NaOH+Cl2��
H2��+2NaOH+Cl2��
| ||
| ||
| 32.5g |
| 6565g/mol |
| VL |
| 22.4L/mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
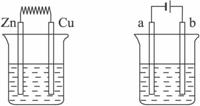
(1)��Aͼ�У�ʹͭƬ��ðH2���ݡ�����Ա�Ҫ���ӣ������Ӻ��װ�ý�________________���缫��Ӧʽ��п�壺_____________________________________________________________��
ͭ�壺____________________________________________________________��
(2)��Bͼ��(a��b��Ϊ���Ե缫)��ʹa������ͭ����b����_______________�����Ա�Ҫ�����Ӻ�װ�ý�_______________���缫��Ӧʽ��a����______________________________��b����______________________________������һ��ʱ���ֹͣ��Ӧ��������Һ����Һ��pH___________ (����ߡ������͡����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ��ɽ�صڶ���ѧ�߶���һ���¿���ѧ�Ծ����������� ���ͣ������
(15��)����ͼ���������Ա�Ҫ�����Ӳ���գ�
(1)��ͼA�У�ʹͭƬ�ϲ���������
�������Ե������ӣ������Ӻ��װ�ý� ���ԭ��ء����ء�����пƬ���� �����������ԭ������ͭƬ�ϵĵ缫��Ӧʽ�� ��
������ӵ�Դ�������Ӻ��װ�ý� ���ԭ��ء����ء���������ͭƬ��Ϊ��װ�õ� �������ӵ�Դ�� ����пƬ�Ϸ����ĵ缫��ӦʽΪ ��
(2)��ͼB�У�ʹa������ͭ��
����a��b�缫��Ϊʯī�缫�����Ա�Ҫ�����ӣ������Ӻ��װ��ӦΪ ���ԭ��ء����ء�������װ�ù���ʱ����Һ���������� ���������ƶ����a����b����������һ��ʱ���ֹͣ��Ӧ��������Һ����Һ������ (���ǿ���������͡����䡱)��
����a��b�缫���Ե������ӣ���a��Ϊͭ�缫����b������Ϊ �缫������ţ���
| A���� | B���� | C��ʯī | D���� E��п |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ�߶���һ���¿���ѧ�Ծ��������棩 ���ͣ������
(15��)����ͼ���������Ա�Ҫ�����Ӳ���գ�
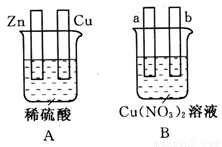
(1)��ͼA�У�ʹͭƬ�ϲ���������
�������Ե������ӣ������Ӻ��װ�ý� ���ԭ��ء����ء�����пƬ���� �����������ԭ������ͭƬ�ϵĵ缫��Ӧʽ�� ��
������ӵ�Դ�������Ӻ��װ�ý� ���ԭ��ء����ء���������ͭƬ��Ϊ��װ�õ� �������ӵ�Դ�� ����пƬ�Ϸ����ĵ缫��ӦʽΪ ��
(2)��ͼB�У�ʹa������ͭ��
����a��b�缫��Ϊʯī�缫�����Ա�Ҫ�����ӣ������Ӻ��װ��ӦΪ ���ԭ��ء����ء�������װ�ù���ʱ����Һ���������� ���������ƶ����a����b������ ����һ��ʱ���ֹͣ��Ӧ��������Һ����Һ������ (���ǿ���������͡����䡱)��
����a��b�缫���Ե������ӣ���a��Ϊͭ�缫����b������Ϊ �缫������ţ���A���� B���� C��ʯī D���� E��п
��װ�ù���ʱ����Һ���������� ���������ƶ����a����b������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��1 3.2�����ĸ�ʴ�ͷ�����ϰ���������棩 ���ͣ������
����ͼ���������Ա�Ҫ�����Ӳ���գ�

��1����Aͼ�У�ʹͭƬ��ðH2���ݡ�����Ա�Ҫ���ӣ������Ӻ��װ�ý� ���缫��Ӧʽ��п�壺 ��ͭ�壺 ��
��2����Bͼ�У�ʹa������ͭ����b������ �����Ա�Ҫ�����Ӻ�װ�ý� ���缫��Ӧʽ��a���� b���� ������һ��ʱ���ֹͣ��Ӧ��������Һ����Һ��pHֵ �����ߡ����͡����䡣��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com