
��15�֣�ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42- �����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ��������NaOH��Һ������仯��ͼ��ʾ:
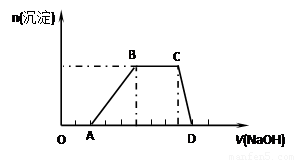
�ɴ˿�֪����1������Һ�п϶����е���������____________________���Ҹ������ӵ����ʵ���֮��Ϊ_____________���϶������е���������___________��������ܺ��е�ij�������ӵij���ʵ�鷽���� ��
��2���ֱ�д��BC��CD�ζ�Ӧ�����ӷ���ʽ����д��BC�βμӷ�Ӧ����������ʵ�����г��õļ�������ڵķ�����BC:________________________________________;
���飺_____________________________________________;
CD:__________________________________________.
��H+��Al3+��NH4+(2��)�� 2��1��3(2��)��Mg2+��Fe3+(2��)����ɫ��Ӧ(2��)��
��NH4++OH- NH3��H2O(��NH4++OH-
=NH3��+H2O)(2��) ; ȡ����δ֪��Һ���Թ��У��μ�����������Һ����ȣ���������д̼�����ζ�����壬�Ҹ�������ʹʪ��ĺ�ɫʯ����ֽ��������֤��ԭ����Һ����NH4+���ڣ������������մ��Ũ����IJ������а�������Ҳ��֤��NH4+���ڣ�(3��)��
NH3��H2O(��NH4++OH-
=NH3��+H2O)(2��) ; ȡ����δ֪��Һ���Թ��У��μ�����������Һ����ȣ���������д̼�����ζ�����壬�Ҹ�������ʹʪ��ĺ�ɫʯ����ֽ��������֤��ԭ����Һ����NH4+���ڣ������������մ��Ũ����IJ������а�������Ҳ��֤��NH4+���ڣ�(3��)��
Al(OH)3+OH- =AlO2-+2H2O(2��)
��������
�����������1������ͼ���֪�����û�г������ɣ�����һ�����������ӡ��������ﵽ���ֵʱ�����������������ƣ������������仯��˵��������NH4+�����ݳ����ܽ�ʱ���ĵ�����������Һ��������ɳ���ʱ���ĵ�����������Һ�����֪��������1�U3����ô˵����ֻ������������û��������þ����������������һ������Al3+����û��Mg2+��Fe3+��������Һ�ĵ����Կ�֪��һ������SO42-���������Ӳ���ȷ�������������ӿ���ͨ����ɫ��Ӧȷ��������ͼ������������������Һ�����������Ϸ�ӦʽH����OH��=H2O��OH����3OH��=Al(OH)3����NH4����OH��=NH3��H2O��֪��H+��Al3+��NH4+�����ʵ���֮��2��1��3��
��2��BC������κ�ǿ��ķ�Ӧ�����Է�Ӧ�Ļ�ѧ����ʽ��NH4++OH- NH3��H2O�����ݸ�ʵ��ԭ�����Լ���NH4������ȡ����δ֪��Һ���Թ��У��μ�����������Һ����ȣ���������д̼�����ζ�����壬�Ҹ�������ʹʪ��ĺ�ɫʯ����ֽ��������֤��ԭ����Һ����NH4+���ڣ������������մ��Ũ����IJ������а�������Ҳ��֤��NH4+���ڣ�����CD�����ܽ����������ģ����Է�Ӧ�ķ���ʽ��Al(OH)3+OH-
=AlO2-+2H2O��
NH3��H2O�����ݸ�ʵ��ԭ�����Լ���NH4������ȡ����δ֪��Һ���Թ��У��μ�����������Һ����ȣ���������д̼�����ζ�����壬�Ҹ�������ʹʪ��ĺ�ɫʯ����ֽ��������֤��ԭ����Һ����NH4+���ڣ������������մ��Ũ����IJ������а�������Ҳ��֤��NH4+���ڣ�����CD�����ܽ����������ģ����Է�Ӧ�ķ���ʽ��Al(OH)3+OH-
=AlO2-+2H2O��
���㣺�������ӹ��桢NH4+���йؼ���
�������������ʵļ���ʱ��Ҫ�������ʵ��������ʺ�������Ӧ��ѡ���ʵ����Լ��ͷ�����ȷ�۲췴Ӧ�е�������������ɫ�ı仯�����������ɺ��ܽ⡢����IJ�������ζ���������ɫ�ȣ������жϡ���������֤���ɡ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?���Ķ�ģ��ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������
��2012?���Ķ�ģ��ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��Һ�п��ܺ���H+��K+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ������ʵ�����NaOH��Һ������仯��ͼ��ʾ���ɴ˿�֪
ij��Һ�п��ܺ���H+��K+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ������ʵ�����NaOH��Һ������仯��ͼ��ʾ���ɴ˿�֪�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
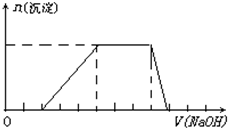 ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ����ı仯��ͼ��ʾ���ɴ˿�֪������Һ��һ�����е���������
ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ����ı仯��ͼ��ʾ���ɴ˿�֪������Һ��һ�����е����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
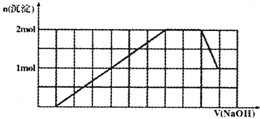 ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������
ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������| A��ԭ��Һ�к��е���������H+��NH4+��Mg2+��Al3+ | B��ԭ��Һ��һ������SO42-��Na+ | C��ԭ��Һ��SO42-�����ʵ�������Ϊ3.5mol | D����Ӧ����γɵ���Һ�к��е�����ΪNa2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
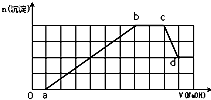 ij��Һ�п��ܺ���H+��NH4+��Mg2+��Fe3+��Al3+��SO42-��HCO3-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ��������NaOH��Һ������仯��ͼ��ʾ������˵����ȷ���ǣ�������
ij��Һ�п��ܺ���H+��NH4+��Mg2+��Fe3+��Al3+��SO42-��HCO3-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ��������NaOH��Һ������仯��ͼ��ʾ������˵����ȷ���ǣ�������| A��d����Һ�к��е�����ֻ��Na2SO4 | B��ԭ��Һ�к��е�Fe3+��Al3+�����ʵ���֮��Ϊ1��1 | C��ab�η��������ӷ�ӦΪ��Al3++3OH-=Al��OH��3����Mg2++2OH-=Mg��OH��2�� | D��ԭ��Һ�к��е������ӱض���H+��NH4+��Al3+�������ܿ϶�Mg2+��Fe3+�е���һ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com