
| 10min | 20min | 30min | 40min | 50min | 60min | |
| 300�� | 0.40 | 0.60 | 0.75 | 0.84 | 0.90 | 0.90 |
| 500�� | 0.60 | 0.75 | 0.78 | 0.80 | 0.80 | 0.80 |

 CH3OH��g����֪��������һ����̼��������n��CO����n��H2��=1��2���з�Ӧ���ɷ���ʽ֪1mol��������1molCO��3molH2��H2����1mol�������������ɶ�����̼��Ӧ�����ݷ���ʽ��֪1molH2�������̼
CH3OH��g����֪��������һ����̼��������n��CO����n��H2��=1��2���з�Ӧ���ɷ���ʽ֪1mol��������1molCO��3molH2��H2����1mol�������������ɶ�����̼��Ӧ�����ݷ���ʽ��֪1molH2�������̼ mol���ݴ˼��㣮
mol���ݴ˼��㣮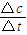 ����v��CH3OH��������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��H2����
����v��CH3OH��������������֮�ȵ��ڻ�ѧ������֮�ȼ���v��H2���� =1.6mol�����ݷ���ʽ���ò���������ת����һ����̼�����ʵ�����ת�������������ʵ�����һ����̼��2����������ת���ʶ�����㣮
=1.6mol�����ݷ���ʽ���ò���������ת����һ����̼�����ʵ�����ת�������������ʵ�����һ����̼��2����������ת���ʶ�����㣮 CH3OH��g����֪��������һ����̼��������n��CO����n��H2��=1��2���з�Ӧ���ɷ�ӦCH4��g��+H2O��g��
CH3OH��g����֪��������һ����̼��������n��CO����n��H2��=1��2���з�Ӧ���ɷ�ӦCH4��g��+H2O��g�� CO��g��+3H2��g����֪1mol��������1molCO��3molH2��H2����1mol�������������ɶ�����̼��Ӧ�����ݷ�ӦCO2��g��+3H2��g��
CO��g��+3H2��g����֪1mol��������1molCO��3molH2��H2����1mol�������������ɶ�����̼��Ӧ�����ݷ�ӦCO2��g��+3H2��g�� CH3OH��g��+H2O��g����֪1molH2�������̼
CH3OH��g��+H2O��g����֪1molH2�������̼ mol������������ԭ�����м����������̼�����Ϊ1mol��
mol������������ԭ�����м����������̼�����Ϊ1mol�� mol=3��1��
mol=3��1�� CH3OH��g����H=-��b-a��kJ/mol��
CH3OH��g����H=-��b-a��kJ/mol�� CH3OH��g����H=-��b-a��kJ/mol��
CH3OH��g����H=-��b-a��kJ/mol��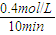 =0.040mol/��L?min��������֮�ȵ��ڻ�ѧ������֮�ȣ�����v��H2��=2v��CH3OH��=2×0.040mol/��L?min��=0.080mol/��L?min����
=0.040mol/��L?min��������֮�ȵ��ڻ�ѧ������֮�ȣ�����v��H2��=2v��CH3OH��=2×0.040mol/��L?min��=0.080mol/��L?min���� CH3OH��g��
CH3OH��g��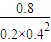 =25
=25 =1.6mol����ת����һ����̼�����ʵ���Ϊxmol����
=1.6mol����ת����һ����̼�����ʵ���Ϊxmol���� CH3OH��g�����ʵ����仯��n
CH3OH��g�����ʵ����仯��n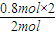 =80%��
=80%��

 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2009?���ڶ�ģ���״��ϳɷ�ӦΪ��CO��g��+2H2��g��?CH3OH��g����ҵ������Ȼ��Ϊԭ�ϣ���Ϊ���Σ�
��2009?���ڶ�ģ���״��ϳɷ�ӦΪ��CO��g��+2H2��g��?CH3OH��g����ҵ������Ȼ��Ϊԭ�ϣ���Ϊ���Σ�| 10min | 20min | 30min | 40min | 50min | 60min | |
| 300�� | 0.40 | 0.60 | 0.75 | 0.84 | 0.90 | 0.90 |
| 500�� | 0.60 | 0.75 | 0.78 | 0.80 | 0.80 | 0.80 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �״��ϳɷ�ӦΪ��CO��g��+2H2��g��?CH3OH��g����ҵ������Ȼ��Ϊԭ�ϣ���Ϊ�����Ʊ��״���
�״��ϳɷ�ӦΪ��CO��g��+2H2��g��?CH3OH��g����ҵ������Ȼ��Ϊԭ�ϣ���Ϊ�����Ʊ��״���| 10min | 20min | 30min | 40min | 50min | 60min | |
| 300�� | 0.40 | 0.60 | 0.75 | 0.84 | 0.90 | 0.90 |
| 500�� | 0.60 | 0.75 | 0.78 | 0.80 | 0.80 | 0.80 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)�״��ϳɷ�ӦΪ��CO(g)+2H2(g) CH3OH(g)
��ҵ������Ȼ��Ϊԭ�ϣ���Ϊ�����Ʊ��״���
(1)�Ʊ��ϳ�����CH4+H2O��g�� CO+3H2��Ϊ����ϳ�����H2������CO��������⣬ԭ������������CO2��CO3+H2=CO+H2O��Ϊ��ʹ�ϳ��������ѣ�������ԭ�����м����������̼�����Ϊ____________________________________��
(2)�ϳɼ״����ٷ�Ӧ���������������仯����ͼ��ʾ��д���ϳɼ״����Ȼ�ѧ����ʽ__________________��
ʵ������1L�ܱ������н���ģ��ϳ�ʵ�顣��lmolCO��2molH2ͨ�������У��ֱ������300���500�淴Ӧ��ÿ��һ��ʱ���������м״���Ũ�����£�
(�������ݵ�λ��mol��L��1)
��300��ʱ��Ӧ��ʼ10�����ڣ�H2��ƽ����Ӧ����Ϊ__________��
��500��ʱƽ�ⳣ��K����ֵΪ___________��
��300��ʱ�����������ݻ�ѹ����ԭ����1��2���������������������£���ƽ����ϵ ������Ӱ����__________(����ĸ)��
a.c(H2)��С b������Ӧ���ʼӿ죬�淴Ӧ���ʼ���
c.CH3OH�����ʵ������� d������ƽ��ʱc(H2)��c(CH3OH)��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ̩���и�����ѧ����ĩ��⻯ѧ�Ծ� ���ͣ������
(10��)�״��ϳɷ�ӦΪ��CO(g)+2H2(g) 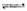 CH3OH(g)
CH3OH(g)
��ҵ������Ȼ��Ϊԭ�ϣ���Ϊ�����Ʊ��״���
(1)�Ʊ��ϳ�����CH4+H2O��g��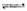 CO+3H2��Ϊ����ϳ�����H2������CO��������⣬ԭ������������CO2��CO3+H2=CO+H2O��Ϊ��ʹ�ϳ��������ѣ�������ԭ�����м����������̼�����Ϊ____________________________________��
CO+3H2��Ϊ����ϳ�����H2������CO��������⣬ԭ������������CO2��CO3+H2=CO+H2O��Ϊ��ʹ�ϳ��������ѣ�������ԭ�����м����������̼�����Ϊ____________________________________��
(2)�ϳɼ״����ٷ�Ӧ���������������仯����ͼ��ʾ��д���ϳɼ״����Ȼ�ѧ����ʽ__________________��
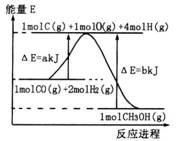
ʵ������1L�ܱ������н���ģ��ϳ�ʵ�顣��lmolCO��2molH2ͨ�������У��ֱ������300���500�淴Ӧ��ÿ��һ��ʱ���������м״���Ũ�����£�
(�������ݵ�λ��mol��L��1)
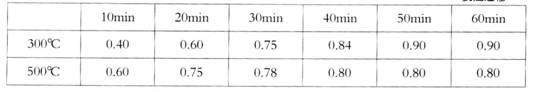 ��300��ʱ��Ӧ��ʼ10�����ڣ�H2��ƽ����Ӧ����Ϊ__________��
��300��ʱ��Ӧ��ʼ10�����ڣ�H2��ƽ����Ӧ����Ϊ__________��
��500��ʱƽ�ⳣ��K����ֵΪ___________��
��300��ʱ�����������ݻ�ѹ����ԭ����1��2���������������������£���ƽ����ϵ ������Ӱ����__________(����ĸ)��
a.c(H2)��С b������Ӧ���ʼӿ죬�淴Ӧ���ʼ���
c.CH3OH�����ʵ������� d������ƽ��ʱc(H2)��c(CH3OH)��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ̩���и������ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
| 10min | 20min | 30min | 40min | 50min | 60min | |
| 300�� | 0.40 | 0.60 | 0.75 | 0.84 | 0.90 | 0.90 |
| 500�� | 0.60 | 0.75 | 0.78 | 0.80 | 0.80 | 0.80 |
 ���������������������£���ƽ����ϵ������Ӱ����______������ĸ����
���������������������£���ƽ����ϵ������Ӱ����______������ĸ����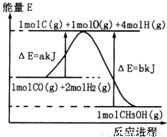
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com