
(10��)������Ԫ��A��B��C��D�У�0.5mol AԪ�ص����ӵõ�6.02��1023�����ӱ���ԭΪ����ԭ�ӣ�0.4g A��������ǡ����100ml 0.2mol/L��������ȫ��Ӧ��Aԭ�Ӻ���������Ŀ��������Ŀ��ȣ�BԪ��ԭ�Ӻ�������������Ŀ�ȵ�һ���1����C����AԪ�ص����Ӷ�1�����Ӳ㣬DԪ�ص�ԭ�Ӻ���ڶ���ȵ�һ���2�����ӡ��ش��������⣺
��1��C�D�Ľṹʾ��ͼΪ ��
��Cͬ�����һ����Ԫ�ص���̬�⻯����ȶ��Ա�HC��________���ǿ������������
��Cͬ�����һ����Ԫ�ص���̬�⻯��ķе��HC��________����ߡ��͡�����
��2��Ԫ��D�����������ĵ���ʽΪ_______��D����̬�⻯��Ķ��ȴ�����_____�ֽṹ��
��3����ҵ��ұ������A�Ļ�ѧ����ʽΪ_____________________________________��
��4����������A��B�õ������Ӳ��뵽����������Һ�п������ԭ��أ�������������_______���û�ѧʽ��д���������缫��Ӧ��___________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ��������ɽ��У��һ��ѧ������������ѧ�Ծ����������� ���ͣ������
�����10�֣�������Ԫ�صĵ���X��Y��Z��ͨ��״���¾�Ϊ��̬����������ת����ϵ����Ӧ������ȥ����
��֪��a������˫ԭ�ӵ��ʷ����У�X���Ӻ����ۼ���ࡣ
b�������к�10�����ӣ��ҷ����к���18�����ӡ�
��1��X�Ľṹʽ�� ��
��2��ʵ���ҿ�����ͼ��ʾװ�ã�ȱ���ռ�װ�ã��г̶ֹ�װ����ȥ���Ʊ��ռ��ס�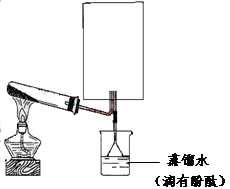
����ͼ�з����ڻ������ƿ�ռ�������װ�ü�ͼ��
���Թ��е��Լ��ǣ��ѧʽ��
���ձ�����Һ����ɫ��Ϊ��ɫ����ԭ���ǣ��õ��뷽��ʽ��ʾ��
��
��д����ҵ�ƼĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ��������ɽ��У��һ��ѧ������������ѧ�Ծ��������棩 ���ͣ������
�����10�֣�������Ԫ�صĵ���X��Y��Z��ͨ��״���¾�Ϊ��̬����������ת����ϵ����Ӧ������ȥ����

��֪��a������˫ԭ�ӵ��ʷ����У�X���Ӻ����ۼ���ࡣ
b�������к�10�����ӣ��ҷ����к���18�����ӡ�
��1��X�Ľṹʽ�� ��
��2��ʵ���ҿ�����ͼ��ʾװ�ã�ȱ���ռ�װ�ã��г̶ֹ�װ����ȥ���Ʊ��ռ��ס�
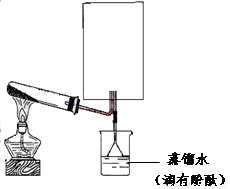
����ͼ�з����ڻ������ƿ�ռ�������װ�ü�ͼ��
�� �Թ��е��Լ��ǣ��ѧʽ��
���ձ�����Һ����ɫ��Ϊ��ɫ����ԭ���ǣ��õ��뷽��ʽ��ʾ��
��
��д����ҵ�ƼĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ֣���и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(10��)�ɶ�����Ԫ����ɵĵ���A��B��C�ͼס��ҡ����������ֻ���������ͼ��ʾ��ת����ϵ����֪C���ܶ���С�����壬���ǵ���ʡ�
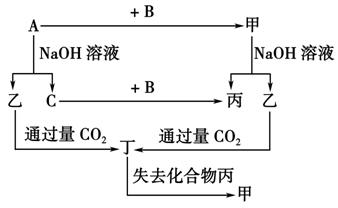
����ͼʾת����ϵ�ش�
(1)д���������ʵĻ�ѧʽ��
A________��B________����________����________��
(2)��ɵ���A��Ԫ�������ڱ��е�λ����_____________�����ĵ���ʽ��
(3)д�����б仯�ķ���ʽ��
��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��__________________________________________
���������CO2��Ӧ�����ӷ���ʽ��__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(10��)�ڶ�����Ԫ���У��ٽ�������ǿ��Ԫ����______����ˮ��Ӧ����ҵķǽ�����_____���ڵؿ��к�����ḻ��Ԫ��λ�ڵ�________���ڵ�________�壬�ؿ��к������Ľ���Ԫ��λ�����ڱ��ĵ�________���ڵ�________�壮
��11��18�ŵ�Ԫ���У���ԭ�Ӱ뾶��С��Ԫ����________�������Ӱ뾶��С����________�������Ӱ뾶��С����________����Ԫ������������м��������ᷴӦ�������ռӦ������������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ֣������ʮ���и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(10��)�ɶ�����Ԫ����ɵĵ���A��B��C�ͼס��ҡ����������ֻ���������ͼ��ʾ��ת����ϵ����֪C���ܶ���С�����壬���ǵ���ʡ�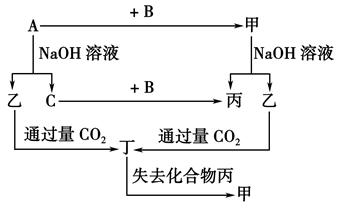
����ͼʾת����ϵ�ش�
(1)д���������ʵĻ�ѧʽ��
A________��B________����________����________��
(2)��ɵ���A��Ԫ�������ڱ��е�λ����_____________�����ĵ���ʽ��
(3)д�����б仯�ķ���ʽ��
��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��_______________ ___________________________
___________________________
���������CO2��Ӧ�����ӷ���ʽ��__________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com