
��12�֣����ԭ����Ӧ���ڹ�ҵ����
��1������ͭ�õ��Ĵ�ͭ�к��������ʣ���п�������ȣ���������Զ���ܴﵽ������ҵ��Ҫ��ҵ�ϳ�ʹ�õ�⾫��������ͭ�ᴿ���ڵ�⾫��ʱ����ͭ�ӵ�Դ �����缫��ӦΪ ����ͭ�� �����缫��ӦΪ ��
��2����ҵ���õ�ⱥ��ʳ��ˮ�ķ������Ƶ��ռ���������������ʱ���ܷ�Ӧ��ѧ����ʽΪ �����ʱ����ľ���ʳ��ˮ��ͨ���ڴ���ˮ�м���ijЩ�Լ�����ȥ���е�Ca2+��Mg2+��Fe3+��SO42-�������ӣ������Լ����Ⱥ�˳�� ���������Լ��Ļ�ѧʽ����
��3��Ϊ�˱���������Ϸ�������Ӧ����ҵ�ϲ������ӽ���Ĥ�����ʳ��ˮ����ͼΪ�����ӽ���Ĥ����ⱥ��ʳ��ˮ 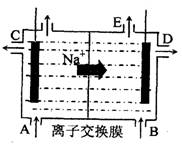 ԭ��ʾ��ͼ���缫��ӦΪ��
ԭ��ʾ��ͼ���缫��ӦΪ��
���� ��
���� ��
����˵������ȷ����
A����E���ݳ���������H2
B.��B�м��뺬����NaOH��ˮ��Һ����ǿ������
C����״����ÿ����22.4L Cl2�������2mol NaOH
D����������������Һ�м��������ᣬ���Իָ������ǰ�����ʵ�Ũ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ԭ����Ӧ���ڹ�ҵ����
��1������ͭ�õ��Ĵ�ͭ�к��������ʣ���п�������ȣ���������Զ���ܴﵽ������ҵ��Ҫ��ҵ�ϳ�ʹ�õ�⾫��������ͭ�ᴿ���ڵ�⾫��ʱ����������ͭ��Һ�����Һ����ͭ�ӵ�Դ �����缫��ӦΪ ��ͨ��һ��ʱ�����Һ��ͭ����Ũ�Ƚ� �������䣬���٣���
��2����ҵ���õ�ⱥ��ʳ��ˮ�ķ������Ƶ��ռ���������������ʱ�������ӷ�Ӧ
ʽΪ �����ʱ����ľ���ʳ��ˮ��ͨ���ڴ���ˮ
����ijЩ�Լ������ṩ���Լ�������Na2CO3��Һ������K2CO3��Һ��NaOH��Һ����BaCl2
��Һ��Ba(NO3)2��Һ�����ᣩ��ȥ���е�Ca2+��Mg2+��Fe3+��SO42-�������ӣ�ѡ���Լ���
���μӵ��Ⱥ�˳��Ϊ ���������Լ��Ļ�ѧʽ����
��3��Ϊ�˱���������Ϸ�������Ӧ����ҵ�ϲ������ӽ���Ĥ�����ʳ��ˮ����ͼΪ����
�ӽ���Ĥ����ⱥ��ʳ��ˮԭ��ʾ��ͼ��
|
A����E���ݳ���������H2
B����B�м��뺬����NaOH��ˮ��Һ����ǿ������
C����״����ÿ����22.4L Cl2�������2mol NaOH
D��������������Һ�м��������ᣬ����
�ָ������ǰ�����ʵ�Ũ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)��ѧԭ����Ӧ���ڹ�ҵ���������ȼҵ�����Ṥҵ��
��1���ȼҵ�õ����豸Ϊ���ӽ���Ĥ���ۣ����ʱ�������� ___________(���Լ�����)����������Ϊ_________________���ѧʽ��,�����ܷ�Ӧ����ʽ____________________________________________________.
��2���Ӵ�����������Ҫ�ֳ���������һ�������ջ�������___________�н��У��ڶ����Ǵ�������������Ӧ�ķ���ʽ___________________________________________,
��������������������ȡ�Ʊ�������������1mol/L����������Һ�к�,��������������Һ�����Ϊ400mL���ų�����ΪQ kJ,д����ʾ�÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ӱ�ʡ�߶��ڶ�ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(10��)��ѧԭ����Ӧ���ڹ�ҵ���������ȼҵ�����Ṥҵ��
��1���ȼҵ�õ����豸Ϊ���ӽ���Ĥ���ۣ����ʱ�������� ___________(���Լ�����)����������Ϊ_________________���ѧʽ��,�����ܷ�Ӧ����ʽ____________________________________________________.
��2���Ӵ�����������Ҫ�ֳ���������һ�������ջ�������___________�н��У��ڶ����Ǵ�������������Ӧ�ķ���ʽ___________________________________________,
��������������������ȡ�Ʊ�������������1mol/L����������Һ�к�,��������������Һ�����Ϊ400mL���ų�����ΪQ kJ,д����ʾ�÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ԭ����Ӧ���ڹ�ҵ����
��1������ͭ�õ��Ĵ�ͭ�к��������ʣ���п�������ȣ���������Զ���ܴﵽ������ҵ��Ҫ��ҵ�ϳ�ʹ�õ�⾫��������ͭ�ᴿ���ڵ�⾫��ʱ����ͭ�ӵ�Դ �����缫��ӦΪ ����ͭ�� �����缫��ӦΪ ��
��2����ҵ���õ�ⱥ��ʳ��ˮ�ķ������Ƶ��ռ���������������ʱ���ܷ�Ӧ��ѧ����ʽΪ �����ʱ����ľ���ʳ��ˮ��ͨ���ڴ���ˮ�м���ijЩ�Լ�����ȥ���е�Ca2+��Mg2+��Fe3+��SO42-�������ӣ������Լ����Ⱥ�˳�� ���������Լ��Ļ�ѧʽ����
��3��Ϊ�˱���������Ϸ�������Ӧ����ҵ�ϲ������ӽ���Ĥ�����ʳ��ˮ����ͼΪ�����ӽ���Ĥ����ⱥ��ʳ��ˮԭ��ʾ��ͼ���缫��ӦΪ��

���� ��
���� ��
����˵������ȷ����
A����E���ݳ���������H2
B����B�м��뺬����NaOH��ˮ��Һ����ǿ������
C����״����ÿ����22.4L Cl2�������2molNaOH
D����������������Һ�м��������ᣬ���Իָ������ǰ�����ʵ�Ũ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com